Vol. 22 - Num. 85
Original Papers
Clinical features and course of disease of community-acquired pneumonia in inpatients
Mercedes Cemeli Canoa, Sara Laliena Aznarb, José Valiente Lozanoc, Berta Martínez Ganuzad, Matilde Bustillo Alonsoe, César García Veraf
aPediatra. CS Valdespartera-Montecanal. Zaragoza. España.
bPediatra. CS Cuarte de Huerva. Zaragoza. España.
cEnfermero. CS Valdespartera. Zaragoza. España.
dPediatra. Servicio de Urgencias Extrahospitalario San Martín. Servicio Navarro de Salud-Osasunbidea. Pamplona. Navarra. España.
eUnidad de Infectología Pediátrica. Servicio de Pediatría. Hospital Universitario Miguel Servet. Zaragoza. España.
fPediatra. CS José Ramon Muñoz Fernández. Zaragoza. España.
Correspondence: M Cemeli. E-mail: cano.mcemeli@salud.aragon.es
Reference of this article: Cemeli Cano M, Laliena Aznar S, Valiente Lozano J, Martínez Ganuza B, Bustillo Alonso M, García Vera C. Clinical features and course of disease of community-acquired pneumonia in inpatients. Rev Pediatr Aten Primaria. 2020;22:23-32.
Published in Internet: 02-03-2020 - Visits: 24116
Abstract
Introduction: community-acquired pneumonia (CAP) remains a common disease in children and is one of the leading causes of death in this age group. The objective of our study was to estimate the incidence of hospital admission due to CAP and describe some of its characteristics.
Material and methods: we conducted a descriptive and retrospective study with inclusion of patients admitted to the Hospital Universitario Infantil Miguel Servet of Zaragoza with a diagnosis of CAP over a 2-year period to describe its clinical, radiological, laboratory, demographic characteristics and associated complications.
Results: we found records for 248 cases of pneumonia; the mean age was 37.6 ± 2.2 months and was significantly higher in cases of typical bacterial pneumonia (41.98 ± 37.46) or atypical pneumonia (73.43 ± 41.28) compared to viral pneumonia (23.30 ± 19.07) (p < 0.0001 and p = 0.0004, respectively). The most common type of pneumonia was of probable pneumococcal aetiology (47.6%; 95 CI: 41.84 to 54.18), and the most frequently identified causative agent was respiratory syncytial virus (34.65%; IC 95: 26.93 to 43.26). The odds ratio of presenting an alveolar radiographic pattern in bacterial pneumonia was 2.98 (95 CI: 1.50 to 5.91; p = 0.0013). The most frequently used antibiotic was intravenous ampicillin (62.87%), with longer duration of treatment in cases of bacterial pneumonia.
Conclusion: CAP requiring hospital admission was most frequent in children aged less than 4 years, with an incidence and associated complications similar to those described in the previous literature. The aetiological diagnosis and subsequent selection of the optimal antibiotic therapy remain challenging.
Keywords
● Community acquired pneumonia ● Hospital ● PediatricsINTRODUCTION
Community-acquired pneumonia (CAP) is the main isolated cause of death in children worldwide. In developed countries, the incidence of CAP in children aged less than 5 years is estimated at 2.6 million cases per year, resulting in 1.5 hospital admissions and approximately 3000 deaths.1,2
For this reason, its high annual incidence, of 30-40 cases per 1000 children younger than 5 years, and its potential severity generate significant concern and lead to a high use of resources. Most studies on CAP are carried out in the hospital setting, and therefore frequently involve the most severe cases. One of the main challenges in the management of children with CAP is establishing an aetiological diagnosis, as it is difficult to detect the involved microorganism with the commonly available laboratory tests. The identification of the causative agent depends on the available diagnostic methods, so in different published studies the aetiology can be determined based on available tests in 40% to 85% of cases.2-4
Complications from pneumonia arise when the infection extends beyond the lung parenchyma into neighbouring tissues or has a more complex course for various possible reasons. This changes the initial course of disease and presents a challenge in its treatment, as there are no standardised criteria on the management of such cases.
The main complications of CAP son: pleural effusion (PE), empyema, pneumothorax, bronchopleural fistula, pulmonary abscess, necrotising pneumonia, pyopneumothorax, bacteraemia or septicaemia. These complications only develop in 1% of cases of pneumonia, although this percentage rises to nearly 40% in patients that require hospitalization.5-7
Pneumonia is the main cause of PE in children; approximately 20% to 40% of those admitted to hospital have PE, and between 0.6% and 2% of those with PE progress to empyema. In recent years there have been epidemiological shifts, with an increase in the prevalence of these complications of pneumonia (the annual incidence of PE has increased from 18 to 42 per 100 000 children, and the incidence of paediatric hospital admission from 0.76 to 3.3 per 100). There have also been changes in the prevalence of different pathogens and serotypes associated with a more rational use of antibiotherapy and changes in vaccination strategy.7,8
Due to all of the above, it is important that we become aware of any epidemiological, clinical, aetiological and antibiotic resistance changes that may occur in CAP in the paediatric population that may require changes in the approach to diagnosis and treatment. Therefore, the aim of our study was to determine the current epidemiological trends in our region and the clinical and disease course patterns in the paediatric population admitted to hospital due to CAP.
MATERIAL AND METHODS
We conducted a retrospective, descriptive and inferential study through the review of health records of patients admitted in the Hospital Universitario Infantil Miguel Servet de Zaragoza (Spain). We included patients admitted with a clinical and radiological diagnosis of pneumonia. The period under study was of 2 natural years (2017-2018), and we included children aged 1 month to 14 years.
Although it is not always necessary to diagnose CAP, we considered chest X-ray the gold standard for diagnosis of CAP. We defined pneumonia as the presence in a previously healthy patient of manifestations compatible with respiratory infection (fever, cough, difficulty breathing, rhinitis) associated with an alveolar radiographic pattern (lobar or segmental consolidation with air bronchogram or pleural effusion in pneumonias of probable bacterial aetiology), or an interstitial pattern (bilateral diffuse perihilar infiltrates, air trapping and atelectasis in case of atypical bacterial or viral pneumonia) or a mixed or indefinite radiographic pattern.
The exclusion criteria were: age less than 1 month or greater than 14 years, primary or secondary immunodeficiency, malignant disease, cystic fibrosis, lung illness (pulmonary sequestration, bronchiectasis, bronchopulmonary dysplasia); encephalopathies associated with a risk of pulmonary aspiration, positive result of tuberculin or purified protein derivative skin test, length of stay greater than 7 days before the onset of symptoms.
The primary variable was the type of pneumonia (bacterial of probable pneumococcal aetiology, atypical bacterial, viral and mixed). The secondary variables under study were demographic variables (age, sex), epidemiological variables (medical history including a history of bronchitis or bronchial hyperresponsiveness (BHR), vaccination status and breastfeeding), clinical features (maximum body temperature and its duration, cough, respiratory rate [RR] assessed based on age according to the criteria of the World Health Organization [WHO]),9 signs of respiratory distress, oxygen saturation and other extrapulmonary manifestations), radiologic features (radiographic pattern, presence and location of pleural effusion and other complications), laboratory characteristics (white blood cell count, neutrophil count, C-reactive protein [CRP]), Mycoplasma pneumoniae IgM antibody test, viral detection tests on nasopharyngeal aspirate (NPA) specimens and treatment (type, route of administration and duration).
Streptococcus pneumoniae was identified by means of culture or by detection of DNA in pleural fluid or blood culture specimens with polymerase chain reaction. The diagnosis of atypical pneumonia was made through detection of M. pneumoniae by means of enzyme-linked immunosorbent assay (ELISA), (based on the reaction of antibodies in the sample to the antigens linked to the polystyrene plate), which allows the quantification of IgM with a sensitivity of 81%-89% in children.1,10
Viral pneumonia was diagnosed by immunofluorescence (IF) on NPA samples, with testing including antigens for the following viruses: respiratory syncytial virus (RSV), influenza A and B, parainfluenza 1,2 and 3, metapneumovirus and adenovirus.
We established the following categories of pneumonia for the analysis: typical bacterial (including infections by Staphylococcus aureus, Streptococcus pyogenes, Bordetella pertussis and probable pneumococcal infection), atypical bacterial (M. pneumoniae and Chlamydia pneumoniae), viral and mixed.
We handled all the collected data in adherence with Organic Law 15/99 on the Protection of Personal Data. The database of the study did not include any personal or personally identifiable data on the patients. We assigned each patient a code that was only known to the research team.
Statistical analysis
We performed a descriptive analysis of the data calculating measures of central tendency and dispersion for quantitative variables (mean, median and standard deviation [SD]); and absolute frequencies and percentages with the corresponding 95% confidence intervals (95 CI). In the inferential analysis, we assessed the association between variables by calculating odds ratios (ORs) and mean differences (MDs) with the corresponding 95 CIs. We assessed statistical significance with the χ2 and Student t tests. We defined statistical significance as a p-value of ≤ 0.05.
RESULTS
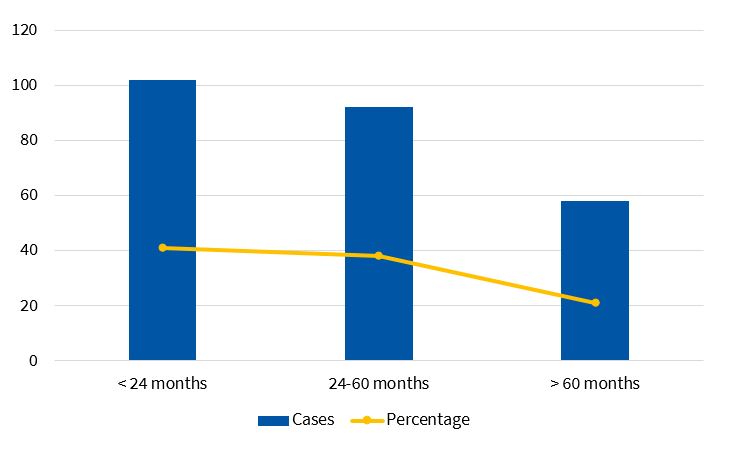
A total of 248 paediatric patients met the inclusion criteria, with a mean age of 37.60 ± 2.20 months. The distribution by sex was nearly uniform (50.92% female, 49.08% male). Figure 1 shows the distribution by age group. Most episodes took place in autumn (36.69%) and winter (26.61%). Typical bacterial pneumonia cases predominated in the autumn and spring, while viral and atypical bacterial pneumonia cases were more frequent in winter and autumn.
Of all patients, 42.80% (95 CI: 36.74% to 49.08%) had a history of asthma or BHR and as many as 4.84% (95 CI: 2.79% to 8.27%) had a previous history of pneumonia. A previous history of BHR was more frequent in patients with viral pneumonias (50.50%; 95 CI: 40.50% to 60.66%) than in patients with typical bacterial pneumonia (38.42%; 95 CI: 30.50% to 46.22%), although the difference was not statistically significant. Similarly, we found a history of BHR in 52.23% of patients with atypical bacterial pneumonia (95 CI: 31.85% a 72.55%), a proportion that was not significantly greater compared to patients with typical pneumonia.
We found a significantly higher mean age in the total of patients with any type of bacterial pneumonia (46.86 ± 39.62 months) compared to patients with viral pneumonia (23.30 ± 19.07 months): MD, 23.56 months (95 CI: 16.10 to 31.02 months, p< 0.0001) (Table 1). We also found a higher mean age in patients with atypical pneumonia (73.43 ± 41.28 months) compared with patients with suspected typical bacterial pneumonia (41.98 ± 37.46 months) (MD: 31.45 months; 95 CI: 14.38 to 48.51; p = 0.0004), and in children with typical bacterial pneumonia compared to children with viral pneumonia (MD: 18.68 months [95 CI: 11.05 a 26.30; p< 0.0001]).
| Table 1. Distribution of type of pneumonia by age group | ||||
|---|---|---|---|---|
| Type of pneumonia/age | <24 months | 24-60 months | >60 months | Total |
| Typical bacterial | 43 (17.34%) | 64 (25.81%) | 19 (7.66%) | 126 (50.81%) |
| Atypical bacterial | 1(0.40%) | 9 (3.63%) | 10 (4.03%) | 20 (8.06%) |
| Viral | 55 (22.18%) | 35 (14.11%) | 3 (1.21%) | 93 (37.50%) |
| Mixed | 3 (1.21%) | 4 (1.61%) | 2 (0.81%) | 9 (3.63%) |
| Total | 102 (41.16%) | 112 (45.14%) | 34 (13.71%) | 248 (100%) |

When it came to vaccination, we found that nearly all children in the case series (99%) were correctly vaccinated against Haemophilus influenzae in adherence to the current official immunisation schedule of Aragon, and 90% correctly vaccinated against pneumococcus (this vaccine was not included in the routine immunisation schedule until year 2016).
Table 2 summarises the most frequent signs and symptoms at the time of diagnosis. The most frequent symptom was fever, present in 85.1% (95 CI: 80.1% to 89.0%), with a mean maximum temperature of 39.04°C ± 0.91°C and a mean duration at the time of diagnosis of 3.88 ± 2.74 days. The mean maximum temperature was significantly higher in patients with viral pneumonia (39.28°C ± 0.77°C) compared to those with typical bacterial pneumonia (38.92°C ± 0.96°C) (MD: 0.36°C; 95 CI: 0.12°C to 0.59°C; p = 0.0031) or with atypical bacterial pneumonia (38.55°C ± 0.94°C) (MD: 0.73°C; 95 CI: 0.34°C to 1.12°C; p = 0.0004).
| Table 2. Frequency distribution of the most frequent signs and symptoms associated with the diagnosis of community-acquired pneumonia | ||
|---|---|---|
| Signs and symptoms | n | Percentage (%) |
| Fever >38°C | 211/229 | 92.10 |
| Cough | 213/244 | 87.20 |
| Tachypnoea* | 87/155 | 56.10 |
| Difficulty breathing | 133/248 | 53.60 |
| Oxygen saturation <92% | 45/241 | 18.60 |
| Gastrointestinal | 29/248 | 11.70 |
| Chest pain | 9/248 | 3.60 |

As for the duration of fever, we only found statistically significant differences between the atypical bacterial pneumonia group (5.72 ± 3.30 days) and the typical bacterial pneumonia group (3.62 ± 2.56 days) (MD: 2.1 days; 95 CI: 0.76 to 3.44; p = 0.0023).
Cough was present in 87% of patients (95 CI: 82% to 90%). It was less frequent in patients with atypical pneumonia (27%, 95 CI: 9% to 46%) compared to patients with typical pneumonia (13%, 95 CI: 7% to 19%). The difference in proportions between the typical and atypical groups was statistically significant (8%; 95 CI, 3% to 14%) (OR: 4.08; 95 CI, 1.25 to 13.34; p = 0.0141).
The analysis of the 155 patients for who there were records of the RR found that 49.03% (95 CI: 41.28% to 56.83%) had tachypnoea. We did not find statistically significant differences in the frequency of tachypnoea between groups, although it was more frequent in patients with viral pneumonia (53.23%; 95 CI: 40.98% to 65.09%) compared to patients with typical bacterial pneumonia (47.22%; 95 CI: 36.13% to 58.60%) or atypical bacterial pneumonia (46.67%; 95 CI: 24.81% to 69.88%).
We did not find differences in the oxygen saturation at admission between patients with typical pneumonia (95.60 ± 3.12), atypical pneumonia (94.52 ± 2.78) and viral pneumonia (95.05 ± 2.90).
The lung auscultation findings were abnormal in 81.45% of patients, most frequently sounds indicative of hypoventilation (49.60%) and crackles (40.32%).
There were other signs and symptoms that were less common but still clinically significant, such as gastrointestinal symptoms (24.60%) (95 CI: 19.65% to 30.32%) and chest pain (4.44%) (95 CI: 2.29% to 7.77%), more frequent in older children.
Table 3 summarises the radiographic findings. The most frequent pattern was alveolar, and PE was found in 19.76% of patients. Stratifying by age group and causative agent, the alveolar pattern was more frequent in patients aged less than 4 years and in pneumonia cases where pneumococcus was the suspected causative agent. The pattern was alveolar in 73.47% of cases of viral pneumonia (95 CI: 64.56% to 82.50%) and 91.30% of cases caused by M. pneumoniae (95 CI: 73.20% to 97.50%). The mean age of patients with an alveolar pattern (39.41 ± 35.97 months) was significantly higher compared to patients with an interstitial pattern (25.71 ± 19.65) (MD: 13.70 months; 95 CI: 1.52 to 25.88, p = 0.0295).
| Table 3. Age distribution of radiographic patterns and other findings of interest such as atelectasis and round pneumonia. | ||||
|---|---|---|---|---|
| Radiographic pattern/age | <24 months | 24-60 months | >60 months | Total (n=248) |
| Alveolar | 78 (37.86%) | 98 (47.57%) | 30 (14.56%) | 206 (83.06%) |
| Interstitial | 8 (57.14%) | 5 (35.71%) | 1 (7.14%) | 14 (5.65%) |
| Mixed | 16 (57.14%) | 9 (32.14%) | 3 (10.71%) | 28 (11.29%) |
| Atelectasis | 6 (28.57%) | 11 (52.30%) | 4 (19.05%) | 21 (8.47%) |

We did not find a statistically significant association of the alveolar pattern with atypical pneumonia (91.30%; 95 CI: 79.80 to 102.80%) compared to typical pneumonia (88.80%; 95 CI: 83.30% to 94.30%). However, the probability of presenting with an alveolar pattern was 2.98 times greater in the overall bacterial pneumonia group (89.20%; 95 CI: 84.21% to 94.22%) compared to the viral pneumonia group (73.50%; 95 CI: 64.77% to 82.23%) (OR: 2.98; 95 CI: 1.50 to 5.91; p = 0.0013), and it was also significantly greater in cases of typical bacterial pneumonia compared to cases of viral pneumonia (OR: 2.86; 95 CI: 1.40 to 5.85; p = 0.0031).
Twenty-one patients had atelectasis, of who 61.90% had pneumonia suspected to be caused by pneumococcus, 23.81% viral pneumonia and the rest pneumonia caused by M. pneumoniae. Parainfluenzavirus type 3 was isolated in 1 of the 3 cases of round pneumonia.
The reasons for hospital admission varied between patients, but the most frequent were pneumonia with hypoxaemia (28.23%), pleural effusion (19.76%), multifocal involvement (11.69%) and failure of oral antibiotherapy (7.26%).
The causative agent was identified in 131 patients (52.82%) (95 CI: 46.61% to 58.95%). The most frequent identified causative agent was RSV (17.74%) (Table 4); S. pneumoniae was detected in 7 cases, and NPA samples tested positive for viruses in 90 patients.
| Table 4. Main causative agents identified | ||
|---|---|---|
| Identified pathogens | n | Percentage |
| Streptococcus pneumoniae | 7 | 2.82% |
| Streptococcus aureus | 1 | 0.40% |
| Streptococcus pyogenes | 2 | 0.81% |
| Bordetella pertussis | 1 | 0.40% |
| Mycoplasma pneumoniae | 24 | 9.68% |
| Chlamydia pneumoniae | 5 | 2.02% |
| Respiratory syncytial virus | 44 | 17.74% |
| Influenza A virus | 21 | 8.47% |
| Influenza B virus | 7 | 2.82% |
| Metapneumovirus | 7 | 2.82% |
| Parainfluenza 1, 2 or 3 virus | 5 | 2.02% |
| Adenovirus | 5 | 2.02% |
| Enterovirus | 1 | 0.40% |
| Mixed | 9 | 3.63% |
| Total | 131 | 52.82% |

We ought to highlight the substantial number of viral pneumonia cases diagnosed in children aged less than 4 years (35.08%). The proportion of cases attributed to M. pneumoniae was also not insignificant (2.42%), nearing the proportion in the group of children aged more than 4 years (4.03%), contrary to the aetiological patterns described historically. Pneumococci were the microorganisms most frequently suspected to be involved in cases of multifocal pneumonia (41.75%), closely followed by viruses (39.81%). Pleural effusion was mainly associated with pneumococcus, and to viruses in a lower proportion. In the cases caused by M. pneumoniae, unifocal involvement was more frequent than multifocal involvement (9.03% vs. 6.80%).
Leucocytosis and neutrophilia were significantly more frequent in the group of typical bacterial pneumonia compared to the viral pneumonia group (Table 5), while elevation of CRP was significantly more frequent in cases of typical pneumonia compared to all other cases. Values or more than 10 mg/dl were detected in 31.05% of patients (95 CI: 25.62% to 37.06%), of which 62.34% corresponded to cases of suspected pneumococcal pneumonia, with a higher incidence of complications.
| Table 5. Results and differences in the white blood cell and neutrophil counts and CPR levels by type of pneumonia | ||||
|---|---|---|---|---|
| Suspected typical bacterial | Atypical bacterial | Viral | Comparison and statistical significance (DM; 95 CI) | |
| WBC count × 1000 (M ± SD) | 17.07 ± 8.69 | 14.83 ± 7.22 | 12.96 ± 7.37 | Typical vs. viral: 4.11; 1.87-6.34 (p = 0.0004) |
| Neutrophil × 1000 (M ± SD) | 11.85 ± 11.04 | 11.04 ± 7.03 | 8.18 ± 6.10 | Typical vs. viral: 3.67; 1.69-5.64 (p = 0.0003) |
| CPR (mg/dl) (M ± SD) | 12.93 ± 15.88 | 6.89 ± 10.30 | 6.16 ± 7.43 |
Typical vs. atypical: 6.04; 0.84-11.24 (p = 0.0239) Typical vs. viral: 6.77; 3.56-9.98 ((p = 0.0001) |

Most hospitalised patients were initially treated with intravenous antibiotherapy (95.56%) (95 CI: 92.23% to 97.51%). In 62.87%, the initial treatment was ampicillin as monotherapy, which failed in 6.04% of patients who required the addition of another drug or switching to another drug, such as amoxicillin-clavulanic acid or cefotaxime. Intravenous amoxicillin-clavulanic acid was used as initial treatment in 13.50% and cefotaxime in 6.75%. A combination of two antibiotics was required by 10.97% of patients. The mean duration of intravenous antibiotherapy was 4.6 ± 3.6 days. Typical bacterial pneumonia was associated with a longer length of stay (6.30 ± 5.08 days) compared to atypical pneumonia (4.78 ± 2.86 days) (MD: 1.52 days; 95 CI: 0.58 to 2.46 days; p = 0.0017) and viral pneumonia (4.97 ± 2.73 days) (MD: 1.33 days; 95 CI: 0.28 to 2.38 days; p = 0.0132).
Some of the patients admitted to hospital were treated with oral antibiotherapy, most frequently azithromycin and amoxicillin. We ought to highlight that as many as 5.91% of patients (95 CI: 3.55% to 9.67%) required treatment with bronchodilators and corticosteroids due to bronchitis. The mean length of stay was 5.63 ± 4.12 days, with a maximum of 27 days, and length of stay was longer in patients with more severe hypoxaemia and those that required drainage of PE.
After discharge, most patients continued to receive oral antibiotherapy, most frequently amoxicillin (56.54%), followed by amoxicillin-clavulanic acid (37.97%) and azithromycin (20.25%); alone or in combination, with a mean duration of 5.6 ± 3.2 days.
DISCUSSION
Our study included 248 patients, and it was consistent with most of the previous literature in that the patients with CAP that most frequently required admission were those younger than 5 years5,8 and in the high proportion of cases with isolation of a virus. In the autumn months, most cases were viral, whereas atypical pneumonia was diagnosed in winter or autumn months with an isolated agent or as coinfection. We ought to highlight the high proportion of patients with a previous history of asthma and BHR that required hospitalization due to viral pneumonia, as well as the substantial number of patients that had bronchopneumonia at the time of diagnosis, which required the addition of bronchodilators to the antibiotherapy regimen. These findings confirm the particular predisposition of these patients to develop more severe respiratory disease, with most cases occurring in children aged less than 2 years.
In our study, we considered the chest X-ray as the gold standard for diagnosis of CAP, and we should mention the challenge that its interpretation may pose in children with associated bronchial involvement. Nevertheless, at the time of this writing it continues to be the sole widely acknowledged gold standard for diagnosis of CAP.
One salient finding was the association of age and the form of pneumonia. Thus, we found that older age was associated more frequently with typical bacterial pneumonia compared to viral pneumonia, and more frequently with atypical pneumonia compared to typical pneumonia, which supports the hypothesis that age may be the best predictor of the type of pneumonia.
As for the clinical presentation, the most frequent symptoms were fever and cough, with fever most frequently having lasted approximately 72 hours at the time of diagnosis. The highest temperature was found most frequently in cases of viral pneumonia compared to cases of bacterial pneumonia (typical or atypical). The mean duration of fever was longer in atypical pneumonia compared to all other forms of pneumonia, which was consistent with most of the previous literature.6-8
On the other hand, cough was absent more frequently in the atypical pneumonia group, although the difference was not statistically significant, probably due to the low number of cases with serologic tests positive for M. pneumoniae.
The findings of lung auscultation were normal in as many as 18.55% of patients, possibly because the duration of illness at the time of the examination was shorter in these patients, a finding that is consistent with most of the previous studies.6-9 On the other hand, tachypnoea has been widely considered the most sensitive and specific sign of radiologically confirmed pneumonia in children, to the extent that some authors have proposed that the absence of tachypnoea could be used to rule out pneumonia with considerable accuracy in febrile children aged less than 5 years, especially those aged less than 2 years.1,6,9 In our study, out of all patients that met the criteria for tachypnoea (49%), 33% had oxygen saturations of 92% or less at diagnosis. We did not find a statistically significant association of tachypnoea with the type of pneumonia in our study, but we found that it was more frequent in patients with viral pneumonia and, although this factor was assessed in each patient, the fact that it was not documented in approximately 100 patients limits our ability to draw conclusions from these data.
One of the most relevant aspects is the radiographic pattern found relative to the type of pneumonia. The interstitial pattern was rare in cases of atypical pneumonia and was more frequent in cases of viral pneumonia. The alveolar pattern was the most frequent one and was associated with older age and bacterial pneumonia overall relative to all other forms of pneumonia and especially with typical pneumonia. Most patients with viral pneumonia exhibited an interstitial or mixed radiographic pattern.
Laboratory tests revealed that the serum level of CRP was significantly higher in cases of typical bacterial pneumonia compared to cases of atypical bacterial pneumonia or viral pneumonia, which was consistent with previous studies. The mean white blood cell and neutrophil counts exhibited relevant differences that could guide the differential diagnosis of typical bacterial pneumonia and viral pneumonia, but not the discrimination of atypical pneumonia, which poses what may currently be the greatest challenge in the differentiation of infection by M. pneumoniae.
Nearly 96% of patients received intravenous antibiotherapy in adherence with current guidelines for management of CAP requiring hospital admission in the paediatric population.1,2,6,7,12,13 The antibiotic used most frequently for initial treatment was ampicillin, followed by amoxicillin-clavulanic acid and cefotaxime, in adherence with the most recent guidelines.10,12,13 A cephalosporin (cefotaxime) as used for initial treatment in 6% of cases. There has been evidence of a high and unjustified use of third-generation cephalosporins in paediatric inpatients with CAP. For instance, a multicentre study conducted in Spain found that 34% of patients received them for initial empirical therapy.13 On the other hand, amoxicillin-clavulanic acid is indicated in patients who have not been correctly vaccinated against H. influenzae, with pneumonia secondary to influenza or, less frequently, in case of suspected aspiration pneumonia, so its use in the case series we present here was more frequent than recommended7 (13.5%). We found that the length of stay was longer in patients with typical bacterial pneumonia compared to all others, which was associated with more frequent or severe complications and the age of the patients.
On the other hand, there is controversy regarding the need to initiate antibiotherapy in young children presenting with symptoms and physical findings compatible with CAP and a non-alveolar radiographic pattern, initial laboratory test results not suggestive of bacterial aetiology and isolation of a virus from NPA samples. In such cases, watchful waiting without antibiotic treatment may be an appropriate approach,1,6,12. although this approach was not used in any of the patients included in our study.
It is well known that CAP in children may be caused by a wide range of aetiological agents, and that determining the agent at play using commonly available techniques may be difficult. In our study, we found that aetiological diagnosis was attempted in cases of viral pneumonia, atypical bacterial pneumonia and in some instances of pneumococcal pneumonia (which proved particularly challenging). The most frequently identified aetiological agent was RSV, consistent with the high prevalence of infection by this virus in younger children and the potential for bronchiolitis to develop in more severe cases of pneumonia by RSV. Aetiological agents atypical for CAP were identified in 5 patients: H. influenzae, S. aureus, S. pyogenes and Bordetella pertussis.
Our findings are consistent with the reports of different studies that found proportions of viral pneumonia of up to 40%12 and of mixed bacterial and viral pneumonia of 20% to 30%.
In our study, cases of mixed infection mainly involved coinfection by 2 viruses, with 3 cases of coinfection with M. pneumoniae and 1 case of coinfection with pneumococcus. Up to 9.68% of patients had infection by M. pneumoniae and 2.02% by C. pneumoniae, a proportion that has been on the rise in the past decade, particularly in children aged less than 2 years, with a frequency of up to 20% reported in some case series.10 The proportion in our sample was not very high compared to other studies, perhaps because these cases of pneumonia are less likely to become complicated or because it is difficult to identify this bacterium by means of serologic tests. The results of IgM antibody tests may be negative in up to 50% of cases (mainly in the first 10-14 days), with antibody titres peaking at 3 weeks and staying positive for up to 2-3 months. Pneumococcus is the agent most likely to be involved in every age group, and in our sample the highest frequency of pneumococcus was found in children aged less than 2 years.
One of the main limitations of our study was the difficulty in establishing the pneumococcal aetiology in children, as NPA samples can be positive simply due to carriage and not be indicative of disease, and urine antigen tests may yield false positive results in healthy children that are carriers or have been recently vaccinated. Since pneumococcal pneumonia causes bacteraemia in very few cases, blood culture is of little use in the aetiological diagnosis. For these reasons, for the purpose of this study, we considered a pneumococcal aetiology probable in cases with compatible clinical and radiographic features with negative results of tests for detection of M. pneumoniae and other bacteria or viruses.
In our study, CAP due to RSV amounted to 17.74% of the cases and was most frequent in children aged less than 2 years. However, given the limitations of the diagnostic tests available for children in our facility, mainly for the detection of pneumococcus, these results must be interpreted with caution.
Although our study included a large sample of cases, it also had limitations, for instance in our ability to obtain complete data, as we collected data retrospectively from patient health records. One of the most important aspects in this area is the establishment of the aetiological diagnosis at the hospital level reflecting the epidemiological reality, with selection of the initial empirical therapy in adherence to the most recent clinical recommendations and guidelines in our region, which are mainly based on the age of the patient, the clinical features and imaging findings.
In conclusion, the initial diagnosis of the type of pneumonia continues to be a challenge in paediatrics, in both the primary care and hospital settings. There is no clinical or radiological parameter that can accurately guide the aetiological diagnosis, and an increasing body of evidence demonstrates how poorly the traditionally recognised patterns of pneumococcal pneumonia as typical and pneumonia of practically any other aetiology as atypical fit the reality of this disease.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article
ABBREVIATION
NPA: nasopharyngeal aspirate · SD: standard deviation · MD: mean difference · PE: pleural effusion · RR: respiratory rate · BHR: bronchial hyperresponsiveness · 95 CI: 95% confidence interval · IF: immunofluorescence · CAP: community-acquired pneumonia · WHO: World Health Organization · OR: odds ratio · CRP: C-reactive protein · RSV: respiratory syncytial virus.
REFERENCES
- Úbeda Sansano MI, Murcia García J, Asensi Monzó MT; Grupo de Vías Respiratorias. Neumonía adquirida en la comunidad. El pediatra de Atención Primaria y la Neumonía. Protocolo del GVR (document P-GVR-8). In: Respirar [accessed 17/02/2020]. Available at www.respirar.org/images/protocolo-neumonia-2017.pdf
- Giménez Sánchez F, Sánchez Marenco A, Battles Garrido JM, López Soler JA, Sánchez-Solís Querol M. Características clínico-epidemiológicas de la neumonía adquirida en la comunidad en niños menores de 6 años. An Pediatr (Barc). 2007;66:578-84.
- Clark JE, Hammal D, Hamptom F, Spencer D, Parker L. Epidemiology of community-acquired pneumonia in children seen in hospital. Epidemiol Infect. 2007;135:262-9.
- Neuman MI, Graham D, Bachur R. Variation in the use of chest radiography for pneumonia in pediatric emergency departments. Pediatr Emerg Care. 2011;27:606-10.
- Rudan I, O'Brien KL, Nair H, Liu L, Theodoratou E, Qazi S, et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. 2013;3:010401.
- Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66 Suppl 2:ii1-23.
- Andrés Martín A, Asensio de la Cruz O, Pérez Pérez G. Complicaciones de la neumonía adquirida en la comunidad: derrame pleural, neumonía necrotizante, absceso pulmonar y pioneumotórax. Protoc diagn ter pediatr. 2017;1:127-46.
- Andrés Martín A, Moreno-Pérez D, Alfayate Miguélez S, Couceiro Gianzo JA, García García ML, Korta Murua J, et al. Etiología y diagnóstico de la neumonía adquirida en la comunidad y sus formas complicadas. An Pediatr (Barc). 2012;76:162.e1-e18.
- The management of acute respiratory infections in children: practical guidelines for outpatient care. In: World Health Organization [online] [accessed 17/02/2020]. Available at https://apps.who.int/iris/handle/10665/41803
- Baer G, Engelcke G, Abele-Horn M, Schaad UB, Heininger U. Role of Chlamydia pneumoniae and Mycoplasma pneumoniae as causative agents of community-acquired pneumonia in hospitalised children and adolescents. Eur J Clin Microbiol Infect Dis. 2003;22:742-5.
- Palafox M, Guiscafre H, Reyes H, Munoz O, Martínez H. Diagnostic value of tachypnoea in pneumonia defined radiologically. Arch Dis Child. 2000;82:41-5.
- Don M, Canciani M, Korppi M. Community-acquired pneumonia in children: what’s old? What’s new? Acta Paediatr. 2010;99:1602-8.
- Borrás C, Novell C, Hernández Bou S, García García JJ. Prescripción antibiótica en los pacientes hospitalizados desde Urgencias. Estudio multicéntrico. An Pediatr (Barc). 2013;79:15-20.