Vol. 26 - Num. 102
Original Papers
Atopic march in a primary care clinic
Silvia Manzanares Santosa, Lucía Tainta Leónb, Alberto Bercedo Sanzc
aPediatra. CS La Carlota. La Carlota. Córdoba. España.
bPediatra. CS La Calzada. Gijón. Asturias. España
cPediatra. CS Los Castros. Santander. Cantabria. España.
Correspondence: S Manzanares . E-mail: silvsms@gmail.com
Reference of this article: Manzanares Santos S, Tainta León L, Bercedo Sanz A. Atopic march in a primary care clinic . Rev Pediatr Aten Primaria. 2024;26:127-36. https://doi.org/10.60147/24a7ffe3
Published in Internet: 20-05-2024 - Visits: 5332
Abstract
Introduction: the prevalence of atopic diseases in childhood has increased in recent years. The aim of this study was to describe the epidemiology of atopic diseases in a primary care paediatrics clinic.
Material and methods: cross-sectional and descriptive study in the children aged 0 to 14 years of a primary care paediatrics caseload (624 children). We collected information on different variables related to atopic diseases. The data were analysed with the software IBM SPSS Statistics version 25. The assessed the association between variables using the chi-square test (considered statistically significant if p <0.05).
Results: Fifty-one percent of the sample had at least one of the atopic diseases. The cumulative prevalence of atopic dermatitis, food allergy, allergic rhinitis and asthma was 27.2%, 3%, 11.7%, and 33%, respectively. Out of all children with atopic dermatitis, 48.8% also had asthma. Of the children with food allergy, 47.4% also developed asthma. Of the patients with allergic rhinitis, 57.5% also presented asthma. We found a statistically significant association between the diagnosis of any of these atopic diseases and a parental history of atopy (p <0.05).
Conclusions: atopic diseases are among the most prevalent disorders encountered in paediatric primary care. There is a strong association between different atopic diseases. A positive family history was significantly associated with the presence of atopic diseases in the paediatric caseload under study.
Keywords
● Allergic rhinitis ● Asthma ● Atopic dermatitis ● Atopic hypersensitivity ● Hypersensitivity ● PrevalenceINTRODUCTION
The term “atopy” refers to the tendency to produce IgE antibodies following exposure to proteins that are inhaled, ingested or that come in contact with the skin surface, producing a clinical picture that varies widely, ranging from asymptomatic sensitization to the development of one or more of the IgE-mediated allergic (atopic) diseases, such as atopic dermatitis (AD), food allergy (FA), asthma, allergic rhinitis/allergic rhinoconjunctivitis (AR/ARC) or even anaphylaxis. Recent studies have found an increase in the prevalence of atopic diseases, estimated at 15-35% in children.1
Atopic dermatitis affects 15-20% of children.2 The prevalence of food allergy peaks at age 1 year at 6-8%.3 In Spain, 20.4% of children aged 6-7 years and 35.2% of adolescents aged 13-14 years report having had symptoms of allergic rhinitis in the past year.4 The prevalence of asthma in the paediatric population in Spain is 15.3% at age 13-14 years and 10.4% at age 6-7 years, with geographical variations, based on data from the Global Asthma Network (GAN)study.5,6
“Atopic march” refers to the progressive character of various atopic manifestations, from the development of atopic dermatitis and food allergy in infants and preschoolers to the development of asthma and allergic rhinitis during school age and adolescence.7
Thus, the presence and severity of atopic dermatitis increases the risk of developing food allergy and the latter, in turn, is a risk factor for the development of asthma and allergic rhinitis.8,9 On the other hand, atopic dermatitis increases the risk of developing asthma and allergic rhinitis.10 In addition, allergic rhinitis is considered a risk factor for the development of asthma11.
The aim of our study was to describe the current prevalence actual and epidemiological characteristics of different atopic diseases in a primary care (PC) paediatrics clinic.
MATERIAL AND METHODS
Cross-sectional descriptive study of the paediatric caseload of a primary care centre (624 children aged 0 to 14 years). The study was conducted through the review of electronic health records. We collected data on the following atopic diseases: food allergy, atopic dermatitis, rhinitis/ allergic rhinoconjunctivitis and asthma. The most relevant variables under study were the presence of atopic disease, family history, treatment, disease severity, diagnostic tests and exposure to tobacco smoke, among others. The analysis was performed using the software IBM SPSS Statistics version 25. We analysed the association between variables by means of the Pearson chi square (χ2), and set the level of statistical significance at p <0.05.
RESULTS
We analysed the electronic health records of 624 children. The mean age was 6.7 years (SD 4.2; median 7 years). The sex distribution was 47.6% male and 52.4% female. We found at least one of the atopic diseases under study documented in 51% of the sample.
The cumulative prevalence for each of the atopic diseases under study was: food allergy 3%, atopic dermatitis 27.2%, allergic rhinitis 11.7% and asthma 33%.
The prevalence of atopic triad (AD, AR and asthma) was 3.5% (6.9% of patients with atopy). Adding food allergy to the triad, the prevalence of the combination was 0.6% (1.3% of patients with atopy).
We analysed the parental history of atopy in the children under study. We found a history of atopic disease in at least one parent in 7.4% of the sample. The parental history of atopy was negative in another 5% of the sample. In the remaining children, we were not able to establish this aspect as it was not documented in the electronic health record.
Of the children with a documented parental history of atopy, 82.6% developed an atopic disease. In contrast, 61.3% of children without a parental history of atopy developed an atopic disease.
We now proceed to break down the information obtained by atopic disease.
Atopic dermatitis
A diagnosis of AD was documented in 27.2% of children and was associated with asthma in 48.8%. We found a statistically significant association between the presence of AD in the child and a family history of atopy involving parents (χ2 8.1, p = 0.02) or siblings (χ2 4.5, p = 0.03).
Food allergy
Three percent of the sample had a food allergy. Most of these patients (89.5%) were younger than 4 years. Of the total children with FA, 47.4% also developed asthma. We included non-IgE mediated cow’s milk protein allergy (CMPA) among the IgE-mediated food allergies on account of its high prevalence and its potential association with future development of atopic diseases.12
Figure 1 presents the distribution of food allergy cases by food group. We found that 10.5% of patients with FA were allergic to 2 foods and 5.3% to 3 foods.
| Figure 1. Absolute frequency and percentage distribution of cases of food allergy per food category. The absolute frequency is shown in the vertical axis and the relative frequency in each bar (percentage of the total detected cases of allergy) |
|---|
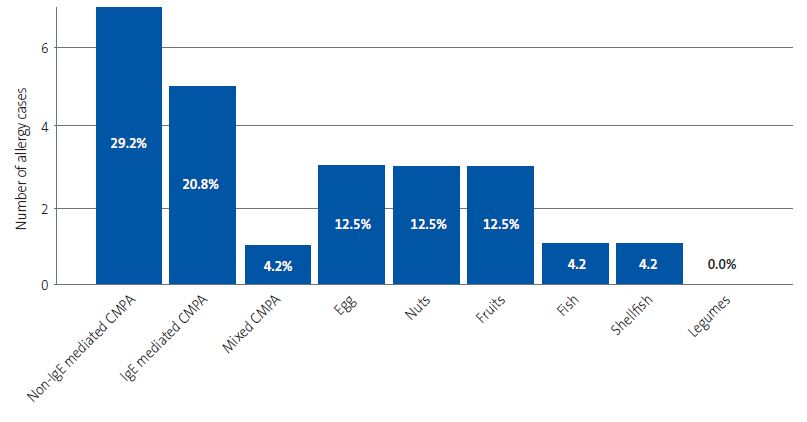 |

We found a statistically significant association between the presence of FA in the child and a parental history of atopy (χ2 16.1, p <0.001).
Rhinitis and allergic rhinoconjunctivitis
Allergic rhinitis was documented in 11.7% of the sample and ARC in 8.7%. The mean age of the patients with AR was 9.7 years (SD 3.1, median 10) and most cases occurred in children aged 7 years and older (60 children, 82.2%).
Of the children with AR, 65.8% had intermittent rhinitis; 31.5% mild persistent rhinitis and 2.7% moderate-severe persistent rhinitis.
In addition, 57.5% of the patients with AR also had asthma and 50% had a history of AD.
We found a statistically significant association between the presence of AR and a parental history of atopy (χ2 15.7, p <0.001). We also found a significant association between severe AR and a family history of atopy in parents or siblings (χ2 11.7, p = 0.02 and χ2 11.4, p = 0.003, respectively).
The most frequent treatment was an oral antihistamine, used in 36.8% of cases. This was followed by treatment with a nasal corticosteroid, prescribed in 32.4% of cases, and an ophthalmic antihistamine, prescribed in 24.5%. Figure 2 provides a graphic representation of these data.
| Figure 2. Treatments prescribed for management of allergic rhinoconjunctivitis. The absolute frequency is shown in the vertical axis and the relative frequency in each bar (percentage of the total treatments prescribed) |
|---|
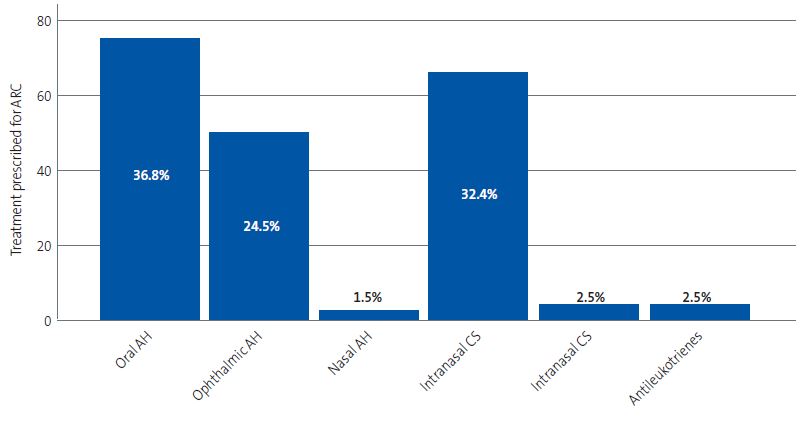 |

Asthma
Thirty-three percent of the sample had a diagnosis of asthma. The cumulative prevalence of asthma by age group was as follows: 7.9% in children aged 0-3 years, 4.6% in children aged 4-5 years and 20.5% in children aged >6 years. The prevalence of active asthma in the past year (defined based on the presence of symptoms and/or the treatment used for asthma control) was 6.7% in children aged 0-3 years, 3% in children aged 4-5 years and 7.5% in children aged >6 years. The mean age of children with asthma was 7.3 years (SD 3.9, median 8 years).
The most frequently used treatments were on-demand salbutamol (58.6%) and inhaled corticosteroids as monotherapy (27.6% of cases). Figure 3 provides a graphic representation of these data.
| Figure 3. Treatments prescribed for management of asthma. The absolute frequency is shown in the vertical axis and the relative frequency in each bar (percentage of the total treatments prescribed) |
|---|
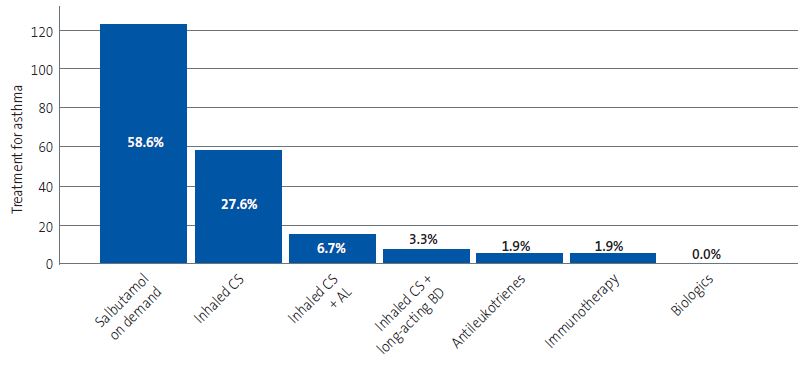 |

We classified patients with asthma based on the treatment required for asthma control. The resulting distribution of the patients can be found in Table 1.
| Table 1. Classification of asthma severity in patients based on the treatment required for adequate symptom control. The table presents the absolute frequency per category, the percentage over the total patients in the sample in the corresponding age group (total %) and the percentage over the total cases of asthma in the corresponding age group (relative %) | ||||||
|---|---|---|---|---|---|---|
| Classification of asthma severity | <6 years | ≥6 years | ||||
| n | Total % | Relative % | n | Total % | Relative % | |
| Intermittent | 38 | 13.9% | 48.7% | 85 | 24.3% | 66.4% |
| Mild persistent | 14 | 5.1% | 17.9% | 28 | 8% | 21.9% |
| Moderate persistent | 22 | 8% | 28.2% | 13 | 3.7% | 10.2% |
| Severe persistent | 4 | 1.5% | 5.1% | 2 | 0.6% | 1.6% |

The prevalence of asthma combined with AR was 6.7% (13.2% of patients with atopy), and the association between these diseases was statistically significant (χ2 22.5, p <0.001). The association between asthma severity and the presence of AR was also significant (χ2 17.9, p <0.001).
We found a statistically significant association between the presence of asthma and a history of atopy in parents or siblings (χ2 14.5, p <0.001 and χ2 36.6, p <0.001, respectively).
In the subset of patients with AR and/or asthma, 16.9% underwent testing to assess for sensitization to respiratory allergens (specific IgE and/or prick test). At the PC level, 11.8% of patients with AR and/or asthma underwent specific IgE tests, but not the prick test as this method was not available at this level of care in the catchment area where the study was conducted. In speciality care (SC) settings, another 5.5% of patients with AR and/or asthma were assessed with prick tests and specific IgE. Also, a spirometry test was performed in 5.5% of patients with AR and/or asthma, in 84.6% of cases in a SC setting.
Fifty percent of patients had developed sensitivity to a single allergen, and the rest to more than one. Most patients were sensitive to olive pollen. The profile of sensitivities detected in our sample can be found in Table 2.
| Table 2. Profile of sensitization to respiratory allergens in patients with allergic rhinitis and/or asthma. The table presents the absolute frequency, percentage over the total sample (total %) and the percentage over the total cases of detected sensitization (relative %) | |||
|---|---|---|---|
| Sensitization to respiratory allergens | n | Total % | Relative % |
| Olive | 21 | 3.4% | 32.8% |
| Grasses | 16 | 2.6% | 25% |
| Alternaria | 11 | 1.8% | 17.2% |
| Dermatophagoides | 8 | 1.3% | 12.5% |
| Cat epithelium | 4 | 0.6% | 6.3% |
| Dog epithelium | 2 | 0.3% | 3.1% |
| London plane | 1 | 0.2% | 1.6% |
| Cypress tree | 1 | 0.2% | 1.6% |

On the other hand, 44% of the sample were exposed to tobacco smoke at home. In a high percentage of patients (25.8%), this aspect was not documented in the health record.
DISCUSSION
The prevalence of atopic diseases has increased rapidly and universally in the past few decades,1 although the possibility that the prevalence is about to reach its possible peak in high-prevalence areas is currently being debated, as demonstrated in the ISAAC-III and GAN epidemiological studies conducted in Spain, which found that the frequency of asthma and AR in school-aged children was stabilising, with a slight increase in adolescentes.4,5
In our study, 51% of the sample had a documented atopic disease, a percentage that was higher compared to other studies published in Spain that were not based in PC health records but on data from the National Health Survey.13
A family history of atopy is the main risk factor for the development of atopic diseases, especially if it involves one or both parents.14 Our findings corroborated it, as 82.6% of children with a documented family history of atopy also developed an atopic disease. However, more than half (61.3%) of children without a family history developed atopic disease, which demonstrates that a negative family history does not rule out the possibility of atopy. We ought to highlight that the family history was not documented in the health record of nearly 88% of patients, so many prevalence values related to this variable may be underestimated. An adequate history-taking that includes the family history is important for the purpose of identifying children at risk.
In the sample under study, there was significant comorbidity between atopic diseases. Of the children with AD, 48.8% also had asthma. Of the children with FA, 47.4% had asthma. Of the patients with AR, 57.5% had asthma and 50% had a history of AD. Thus, a combined approach to the management of atopic diseases is advisable to ensure their adequate treatment and control treatment and control, given the close association between them.
Allergic rhinoconjunctivitis was the main presenting complaint of children referred to an allergy unit based on the Alergológica 2015 nationwide study, and AR was the most frequent respiratory disease and an important risk factor for asthma.2 In Spain, 20.4% of children aged 6-7 years and 35.2% of adolescents aged 13-14 years reported symptoms of AR in the past year.11,15 We found a lower prevalence in our study, of 11.7%. Some possible reasons for this difference are the lack of a medical diagnosis and the low frequency of paediatric visits due to rhinitis, probably on account of the seasonality of the symptoms and their mild nature.
As regards the treatment of AR, nasal corticosteroids (NCs) are the most effective medication and other drugs should be considered second-line options in the absence of an adequate response to first-line treatment.11 In this case, the addition of an oral or an intranasal antihistamine would be indicated, with the latter route being more effective against nasal congestion and having a quicker onset of action.11 In our study, the most frequently prescribed treatment was an oral antihistamine (36.8% of patients with AR), followed by NCs (32.4%). We ought to highlight the low frequency of prescription of intranasal antihistamines (1.5%), which was even exceeded by the prescription of montelukast (2.5%), which has similar or inferior effects than those of antihistamines.
When it came to bronchial asthma, the Alergológica 2015 report ranked it second in frequency among the reasons for consultation in allergy clinics after AR, which was corroborated by the data for the prevalence of asthma in Spain of the GAN in the paediatric population, which was 15.3% at age 13-14 years and 10.4% at age 6-7 years, with variations between geographical areas.2
Our study found a cumulative prevalence of asthma of 33% for all age groups, with a prevalence of active asthma of 17.2% (7.5% in children aged more than 6 years). The observed cumulative prevalence of asthma was high and also greater than the prevalence of AR (11.7%), in contrast to the findings of other recent studies,2,4,5 but could be explained by the confounding effect of the diagnosis of wheezing in infants and children aged up to 5 years documented in the health records.
In this regard, when it came to asthma in infants and children aged less than 3 years, diagnosing it based on the occurrence of more than 3 episodes of wheezing, and despite the current controversy on the definition of asthma in these age groups, we found that 6.7% of these children had a diagnosis of asthma (less than the 7.7% found in children under 2 years in other studies conducted within a small geographical distance in 2002). Few recent studies have analysed the prevalence of asthma in the first years of life, with the exception of the International Study of Wheezing in Infants, in which 1 in 3 infants was found to have at least 1 episode of wheezing and 1 in 7 or 8 infants had 3 or more episodes, independently of the place of residence.16,17
On the other hand, our study corroborated the appropriate prescribing of pharmacological treatment for asthma based on severity, in adherence to the recommendations provided in asthma clinical practice guidelines.18,19
Allergy tests were only performed in 16.9% of patients with AR and/or asthma. At the PC level, only specific IgE tests were performed, as the prick test was not available, despite being the method of choice for the initial assessment of allergic diseases. The allergens to which patients were sensitised most frequently in the caseload under study (olive pollen, grasses, Alternaria and dust mite) were consistent with the allergic sensitization map for the region of Andalusia.2 Knowing the sensitization profile of atopic patients is essential, as is the availability of the tests required for the diagnosis of allergies at the PC level, as diagnosis allows the implementation of adequate educational and environmental control interventions.
When it came to spirometry, the test was only performed in 5.5% of patients with AR and/or asthma, and in most of these patients (84.6%) it was performed in a speciality care setting. These findings evince the need of using spirometry at the PC level as a complementary test to confirm the diagnosis of asthma and enhance the management and followup of patients with asthma. It is also a very useful tool to complement the health education of children with asthma and can contribute to improve the psychosocial wellbeing of the parents.20
We found that nearly half of the caseload (44%) was exposed to tobacco smoke at home, a percentage that could have been underestimated, as tobacco use in the home was not documented in the health records of 25.8% of the patients. The effects of passive smoking and respiratory illnesses during childhood is well documented, leading to an increase in respiratory infections, otitis and asthma in children.21,22 Therefore, the PC paediatrician plays an essential role in the management of passive smoking, documenting it in the health record of the patient and enabling the identification of patients at risk .
The PC paediatrician is a key figure in the tertiary prevention of allergic diseases, that is, once they occur (which is when the patient tends to seek care). The provider must implement strategies to attempt to prevent the development of symptoms, such as measures to avoid exposure to allergens and tobacco smoke, patient and family education or initiation of pharmacological treatment for disease control.1
On the other hand, asthma and associated allergic comorbidities have a negative impact on the quality of life of children and adolescents and their families, with a poorer perception of physical and mental health, restrictions to activities of daily living and an increased use of health care services, based on data from the National Health Survey.13 Thus, specific care protocols must be created at the PC level to improve the diagnosis, treatment and followup of these patients.
There were limitations to our study. It was conducted in a single centre in a disadvantaged population. Therefore, the sample was not representative of the entire paediatric population. Due to its retrospective design, there were also limitations in data collection. Since it was cross-sectional, we were not able to infer causality.
Lastly, despite the limitations of our study, its findings can help reflect on the approach to these diseases at the PC level.
CONCLUSION
Half of the PC paediatrics caseload under study (51%) had some form of allergic disorder, with a cumulative prevalence of diagnosis of AD, FA and AR of 27.2%, 3% and 11.7%, respectively. In the case of asthma, the cumulative prevalence in children aged > 6 years was 20.5%, and 7.5% had active asthma in the past year. The significant association between allergic diseases in the patients and the family history of atopy calls for an active assessment for the identification of any of these diseases in patients and their families, and a combined approach to the management of any identified atopic diseases to ensure appropriate treatment and control. On the other hand, the treatments prescribed for children with asthma aged more than 6 years were appropriate for the disease severity, while in the case of AR, adherence to treatment recommendations in clinical guidelines was poorer. The capabilities for the diagnosis of allergic diseases and asthma must be increased in PC settings, in light of the low frequency of performance of allergy tests and spirometry. In conclusion, a comprehensive approach to the management of allergic diseases at the PC level is a goal that must be pursued, given the impact of these diseases on the quality of life of children and adolescents as well as their families.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article.
AUTHORSHIP
All authors contributed equally to the development of the published article.
ABBREVIATIONS
AD: atopic dermatitis · AR: allergic rhinitis · ARC: allergic rhinoconjunctivitis · CMPA: cow’s milk protein allergy · FA: food allergy · GAN: Global Asthma Network · INAH: intranasal antihistamine · NC: nasal corticosteroid · OAH: oral antihistamine · PC: primary care· SC: speciality care.
REFERENCES
- Ridao Redondo M, Fernández Alonso JE. Orientación diagnóstica de alergia a través de la historia clínica. ¿Cuándo se debe sospechar etiología alérgica? Evolución de la enfermedad alérgica en la edad pediátrica. Protoc Diagn Ter Pediatr. 2019;2:1-15. In: AEP [online] [accessed 09/05/2024]. Available at www.aeped.es/sites/default/files/documentos/01_orientacion_diagnostica.pdf
- Ojeda P, Ibáñez MD, Olaguibel JM, Sastre J, Chivato T; investigators participating in the National Survey of the Spanish Society of Allergology and Clinical Immunology Alergológica 2015. Alergológica 2015: A National Survey on Allergic Diseases in the Spanish Pediatric Population. J Investig Allergol Clin Immunol. 2018 Oct;28(5):321-9. https://doi.org/10.18176/jiaci.0308
- Escarrer Jaume M, Juliá Benito JC, Quevedo Teruel S, Prieto del Prado A, Sandoval Ruballos M, Quesada Sequeira F, et al. Cambios en la epidemiologia y en la práctica clínica de la alergia mediada por IgE en pediatría. An Pediatr. 2021 Jul;95(1): 56.e1-56.e8. https://doi.org/10.1016/j.anpedi.2021.04.014
- Bercedo Sanz A, Martínez-Torres A, López Silvarrey Varela A, Pellegrini Belinchón FJ, Aguinaga Ontoso I, González Díaz C, et al. Prevalence and time trends of symptoms of allergic rhinitis and rhinoconjunctivitis in Spanish children: Global Asthma Network (GAN) study. Allergol Immunopathol. 2023;51(5):1-11. https://doi.org/10.15586/aei.v51i1.711
- Bercedo Sanz A, Martínez-Torres A, González Díaz C, López-Silvarrey Varela A, Pellegrini Belinchóng FJ, Aguinaga-Ontoso I, et al. Grupo GAN España. Prevalencia y evolución temporal de síntomas de asma en España. Estudio Global Asthma Network (GAN). An Pediatr (Barc) 2022;97:161-7. https://doi.org/10.1016/j.anpedi.2021.10.007
- Moral Gil l, Asensi Monzó M, Juliá Benito JC, Ortega Casanueva C, Paniagua Calzón NM, Pérez García MI, et al. Asma en pediatría: Consenso REGAP. An Pediatr (Barc), 2021; 95: 125.e1-125.e11. https://doi.org/10.1016/j.anpedi.2021.02.009
- Yang l, Fu J, Zhou Y. Research Progress in Atopic March. Front Immunol. 2020;11:1907. https://doi.org/10.3389/fimmu.2020.01907
- Tran MM, Lefebvre DL, Dharma C, Dai D, Lou WYW, Subbarao P, et al. Predicting the atopic march: results from the Canadian Healthy Infant Longitudinal Development Study. J Allergy Clin Immunol. 2018;141(2):601-7. https://doi.org/10.1016/j.jaci.2017.08.024
- Paller AS, Spergel JM, Mina-Osorio P, Irvine AD. The atopic march and atopic multimorbidity: Many trajectories, many pathways. J Allergy Clin Immunol. 2019;143(1):46-55. https://doi.org/10.1016/j.jaci.2018.11.006
- Tsuge M, Ikeda M, Matsumoto N, Yorifuji T, Tsukahara H. Current Insights into Atopic March. Children (Basel). 2021;8(11):1067. https://doi.org/10.3390/children8111067
- Bercedo Sanz A, Guerra Pérez MT, Callén Blecua MT. Rinitis alérgica. El Pediatra de Atención Primaria y la rinitis alérgica. Protocolos del GVR (publicación P-GVR-6) [online] [accessed 09/05/2024]. Available at www.aepap.org/sites/default/files/documento/archivos-adjuntos/rinitis_alergica_p_gvr_6_2016.pdf
- Bahceci S, Töz PK, Celik FC, Can D. A different starting line for allergic march: food protein-induced allergic proctocolitis. Allergol Immunopathol (Madr). 2023;51(4):40-5. https://doi.org/10.15586/aei.v51i4.872
- González de Paz l, Valdesoiro Navarrete l, Roma J, Blat Guimerà E, Benavent Areu J, Bartra J, et al. Prevalence and Impact of Asthma and Allergy on Daily Life, Health Outcomes and Use of Healthcare Services in Children: A Population-Based Study. Arch Bronconeumol. 2023;59(8):481-7. https://doi.org/10.1016/j.arbres.2023.05.005
- Crnković HT, Bendelja K, Šimić Klarić A, Tomić Rajić M, Drkulec V, Aberle N. Family history and cord blood eosinophil count as predictors for atopic manifestations. Cent Eur J Public Health. 2019;27(4):267-71. https://doi.org/10.21101/cejph.a5601
- Strachan DP, Rutter CE, Asher MI, Bissell K, Chiang CY, El Sony A, et al; Global Asthma Network Phase I Study Group. Worldwide time trends in prevalence of symptoms of rhinoconjunctivitis in children: Global Asthma Network Phase I. Pediatr Allergy Immunol. 2022;33(1):e13656. https://doi.org/10.1111/pai.13656
- Bercedo Sanz A, Lastra Martínez l, Pellegrini Belinchon J, Vicente Galindo E, Lorente Toledano F, García Marcos l. Wheezing and risk factors in the first year of life in Cantabria, Spain. The EISL study. Allergol Immunopathol (Madr). 2015;43:543-52. https://doi.org/10.1016/j.aller.2014.09.001
- Pellegrini Belinchon J, Miguel Miguel G, Dios Martín B, Vicente Galindo E, Lorente Toledano F, García Marcos l. Study of wheezing and its risk factors in the first year of life in the Province of Salamanca, Spain. The EISL Study. Allergol Immunopathol (Madr). 2012;40:164-71. https://doi.org/10.1016/j.aller.2011.03.014
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. In: GINA [online] [accessed 09/05/2024]. Available at https://ginasthma.org/2023-gina-main-report/
- Grupo de trabajo de la Guía Española para el Manejo del Asma. GEMA 5.3. 2023. In: SEMG [online] [accessed 09/05/2024]. Available at www.semg.es/index.php/consensos-guias-y-protocolos/399-gema-5-3-guia-espanola-para-el-manejo-del-asma
- Boonjindasup W, Marchant JM, McElrea MS, Yerkovich ST, Masters IB, Chang AB. Does routine spirometry impact on clinical decisions and patient-related outcome measures of children seen in respiratory clinics: an open-label randomised controlled trial. BMJ Open Respir Res. 2023;10(1). https://doi.org/10.1136/bmjresp-2022-001402
- Duelo Marcos M, Moneo Hernández MI; Grupo de Vías Respiratorias. El pediatra de AP y el tabaco. Protocolo del GVR (publicación P-GVR-8) [online] [accessed 09/05/2024]. Available at www.aepap.org/sites/default/files/documento/archivos-adjuntos/pediatra-y-tabaquismo-2018.pdf
- Córdoba García R, García Sánchez N, Suárez López de Vergara RG, Galván Fernández C. Exposición al humo ambiental de tabaco en la infancia. An Pediatr (Barc). 2007;67(2):101-3. https://doi.org/10.1016/S1695-4033(07)70568-4
Comments
This article has no comments yet.














