Vol. 19 - Num. 76
Original Papers
Impact of the underfunding of the pneumococcal vaccine in a low-income population
Adrián Abbasi Péreza, María Aparicio Rodrigob, Carlos Ochoa Sangradorc
aEstudiante de Medicina. Universidad Complutense. Madrid. España.
bPediatra. CS Entrevías. Facultad de Medicina. Universidad Complutense de Madrid. Madrid. España.
cServicio de Pediatría. Hospital Virgen de la Concha. Zamora. España.
Reference of this article: Abbasi Pérez A, Aparicio Rodrigo M, Ochoa Sangrador C. Impact of the underfunding of the pneumococcal vaccine in a low-income population. Rev Pediatr Aten Primaria. 2017;19:329-36.
Published in Internet: 26-10-2017 - Visits: 11192
Abstract
Introduction: in June 2010, the Community of Madrid introduced the 13-valent pneumococcal conjugate vaccine in the childhood vaccination schedule. Due to budgetary restrictions, public funding for the vaccine was withdrawn between July 2012 and March 2015. Our objective was to assess the impact of defunding on vaccination coverage and on the incidence of acute otitis media, pneumonia and invasive pneumococcal disease in a low-income population, comparing these figures with those reported for the whole region.
Methods and materials: Retrospective cohort study of pneumococcal disease and vaccination coverage in children born between May 2012 and October 2014 served by the Entrevías Primary Care Centre.
Results: We found a decrease in vaccination coverage (66%, 95% CI: 57.3 to 71.4%) compared to the average in the Community of Madrid (77%). There were no cases of invasive pneumococcal disease, and the incidence of pneumonia and acute otitis media was independent of vaccination status. The only factor associated to the incidence of acute otitis was attendance to childcare centres.
Conclusions: The defunding of the conjugate vaccine against thirteen pneumococcal serotypes caused a decrease in vaccination coverage in children from a low-income population. In this study, we did not find an increase in the incidence of pneumococcal disease, which may be due to the persistence of the herd effect or to an insufficient sample size.
Keywords
● Otitis media ● Pneumococcal infections ● Pneumococcal vaccines ● Pneumonia, pneumococcal ● Vaccination coverageINTRODUCTION
Streptococcus pneumoniae, known as pneumococcus, is a gram-positive coccus of which more than 90 serotypes are known. Its sole reservoir is the nasopharynx, especially of individuals aged less than 4 years, with colonization being less frequent in adults. Pneumococcus can cause local infections of mucosal tissues (pneumonia, acute otitis media [AOM] or sinusitis) or can spread to the bloodstream, resulting in invasive pneumococcal disease (IPD) (pneumococcal pneumonia, meningitis, bacteraemia [most frequent form of IPD] or sepsis).1
The first pneumococcal vaccine was the polysaccharide vaccine licensed in 1977 in the United States for patients aged more than 2 years at risk of pneumococcal infection. This vaccine elicited a poor immune response in individuals aged less than 2 years, in whom the incidence of IPD is higher.
In 2000, clinical trials for the first pneumococcal conjugate vaccine (PCV), which included the 7 most prevalent serotypes (PCV-7), were completed.2 This vaccine exhibited an excellent effectiveness against IPD caused by vaccine serotypes.2 Its effectiveness against non-invasive forms of disease, such as AOM and pneumonia, was considerably lower.3
Before the introduction of the PCV-7, the incidence of IPD in Spain ranged between 60 and 70 cases per 100 000 children aged less than 2 years. Routine vaccination with PCV-7 achieved a reduction in its incidence, but gave rise to an increase in invasive disease caused by other serotypes (19A, 1, 7F, 3 and 6A).
In 2010, the 13-valent pneumococcal conjugate vaccine (PCV-13), which includes the PCV-7 serotypes and six other (1, 3, 5, 6A, 7F and 19A) entered the Spanish market. The Community of Madrid (CM) started to vaccinate with PCV-13 instead of PCV-7 in June 2010. Since then, the incidence of IPD in children aged less than 15 years has decreased from 17 to 7.7 cases per 100 000 inhabitants (a 57% reduction). In this same period, the number of cases of empyema, bacteraemic pneumococcal pneumonia and pneumococcal meningitis dropped by 46%, 71% and 55% respectively. Its use in other countries has achieved similar results.4-6
In July 2012, due to budgetary restrictions, the PCV-13 was withdrawn from the routine immunisation schedule of the CM, and only maintained for vaccination of at-risk groups until January 2015, when it was reintroduced. According to the Boletín Epidemiológico (Epidemiological Report) of the CM, during this period the vaccination coverage decreased from 99.8% in 2011 to 77.1% in 2013; the data were not stratified by basic health zone.5 Although the data recently published by the CM suggest that there has not been an overall increase in the incidence of IPD, we do not know the impact that the removal of the vaccine may have had in low-income areas.4
The aim of this study was to assess the impact of eliminating funding for the PCV-13 vaccine on vaccination coverage and the incidence of pneumococcal disease in a basic health zone of the CM corresponding to a very low-income area, and to compare these data with the official data for the entire CM.
MATERIALS AND METHODS
We obtained the approval of the Comisión Local de Investigación Sureste (Local Research Committee of Southeast Madrid), and we adhered to the established protocol for accessing data from electronic health records for the purpose of research.
The study included all patients born between May 2012 and October 2014 assigned to the Paediatrics caseload of the Entrevías Primary Care Centre (PCC) of Madrid, Spain. We chose this period because it corresponded to the cohort of children for which the CM removed funding for the PCV-13 (from July 2012 to January 2015). We obtained the list of patients from the Cibeles database, which has records for the entire population of individuals in the CM who hold a public health card (with the right to access public health care services). For this population, we determined the coverage for the PCV-13 vaccine and the incidence of pneumococcal disease, invasive as well as non-invasive (AOM and pneumonia).
We collected the data from the electronic health records of the AP-Madrid® primary care database. For each patient included in the Cibeles register, we collected the date of birth, gestational age at birth, sex and childcare centre attendance. In regards to vaccination, we collected data on the received doses of PCV-13 and the dates of their administration. We only took into account doses administered between July 1, 2012 and December 31, 2014. We considered that children had been vaccinated if they had received the vaccine doses that corresponded to their age based on the summary of product characteristics for the vaccine (Prevenar13®), including patients who received the doses at the recommended ages for vaccination while the vaccine was publicly funded, as well as those vaccinated with catch-up doses at older ages. We considered that children had not been vaccinated if they received no doses of PCV-13 or had an incomplete vaccination status according to the specifications of the summary product characteristics.
We collected data for the presence or absence of underlying diseases that could make patients more vulnerable to pneumococcal disease: chronic lung disease, cyanotic congenital heart disease, Down syndrome, diabetes mellitus, chronic liver disease, subarachnoid space fistulae, asplenia and immunosuppression.
We collected data for episodes of disease coded as AOM or suppurative otitis media, including the date of diagnosis, care setting at diagnosis (primary care/hospital), otoscopic findings suggestive of AOM, presence or absence of fever and clinical manifestations compatible with otitis. We defined AOM as the symptomatic presence of discharge (usually mucopurulent) in the middle ear (consensus diagnostic criteria for AOM).7
We also collected data for episodes coded as pneumonia, including the date of diagnosis, care setting at diagnosis (primary care/hospital) and type of diagnosis (radiological/clinical/laboratory). We defined pneumonia as an acute lower respiratory tract infection lasting fewer than 14 days, acquired in the community, manifesting with cough or breathing difficulties and with radiological evidence of acute pulmonary infiltrates (consensus diagnostic criteria for community-acquired pneumonia).8 We also recorded information on episodes compatible with IPD (meningitis, septicaemia).
Furthermore, we retrieved data for the same population from the Minimum Basic Data Set (MBDS) records for hospital discharges or outpatient surgeries in the CM through the consult@web Primary Care software application, searching for the following codes for invasive pneumococcal disease included in the ICD-9-MC: 481 (pneumococcal pneumonia), 320.1 (pneumococcal meningitis), 038.2 (pneumococcal septicaemia) and 567.1 (pneumococcal peritonitis), entered as the primary diagnosis or the first secondary diagnosis, and also for codes 382.0, 382.4, 382.9 (acute otitis media), 482.9 (bacterial pneumonia, unspecified), 485 (bronchopneumonia, organism unspecified) and 486 (pneumonia, organism unspecified). We used the same application to obtain a list of the episodes recorded between September 2012 and January 2015 in the primary care electronic health records (AP-Madrid®) associated with pneumococcal infection and with the following CIPC codes: pneumonia (R81), otitis (H71), meningitis (N71), sepsis; as well as episodes coded with other diagnoses but whose description included any of the diseases mentioned above. We compared these data with the data included in the individual health records of each patient to prevent missing data.
We compared the PCV-13 vaccination coverage at the Entrevías PCC with the PCV-13 vaccination coverage in the entire CM for the period under study. We also compared the incidence of disease potentially caused by pneumococcus in unvaccinated versus vaccinated patients, taking into account the number of doses of vaccine received prior to diagnosis.
We performed a descriptive analysis with calculation of absolute and relative frequencies for qualitative variables and measures of central tendency and dispersion for quantitative variables. We calculated the confidence intervals of the main measurements. We assessed the association between qualitative variables with the χ2 or Fisher exact tests. Furthermore, we performed survival analysis to estimate the time elapsed to the onset of the first otitis episode by the Kaplan-Meier method and long-rank test (results not shown).
We calculated the sample size needed to estimate the proportion of vaccinated individuals with an accuracy of 10% and a 95% confidence interval (95 CI), for an expected proportion of 50% (the most unfavourable possibility), which was of 96 subjects.
RESULTS
We selected an initial sample of 201 children. We excluded 7 patients who did not have electronic health records. A total of 194 patients were included in the final analysis. The mean age of the sample was 17 months (95 CI: 16 to 19), with a median of 18 months and a range of 0 to 30 months.
Sixty-six percent of patients were correctly vaccinated (at the scheduled age or with delays in relation to the official immunisation schedule). The 95 CI for the vaccination coverage was 57.3% to 71.4%. We found no differences in terms of sex, gestational age or attendance to childcare centres between the vaccinated and unvaccinated groups of children, although vaccinated children were younger compared to unvaccinated children (Table 1).
| Table 1. General characteristics of patients vaccinated with PCV-13 and unvaccinated patients | |||
|---|---|---|---|
| Vaccinated (n = 126) | Incomplete or no vaccination (n = 65) | ||
| Sex, male / female | 63/63 (50%) | 36/29 (55%) | P = .48 |
| Months of age: mean (standard deviation) | 16.22 (8.77) | 19.6 (9.65) | P = .016 |
| < 37 weeks’ gestational age | 11/126 (5%) | 50/65 (4%) | P = 1.000 |
| Enrolment in childcare (out of 170) | 49/119 (41%) | 19/51 (37%) | P =.731 |

There were no cases of IPD. There were a total of 48 cases of AOM in our sample during the period under study: 33 cases in vaccinated children (26.2% of this subset) and 15 cases in unvaccinated children (23.1% of this subset). The mean age was 13 months (95 CI: 11 to 14). The age at onset of AOM was 3 months. Of all cases, 35.4% (17 cases) occurred before vaccination, 33.3% (16 cases) after the primary series (3 doses administered in the first year of life) and before the booster dose, and 31.3% (15 cases) after vaccination was completed (Figure 1). We found no significant differences between the groups. The incidence of otitis was not associated with vaccination status, gestational age or sex.
| Figure 1. Prevalence of AOM in children vaccinated with the 13-valent pneumococcal conjugate vaccine (before vaccination, after primary vaccination, after complete vaccination according to their age) and children who were unvaccinated during the period under study |
|---|
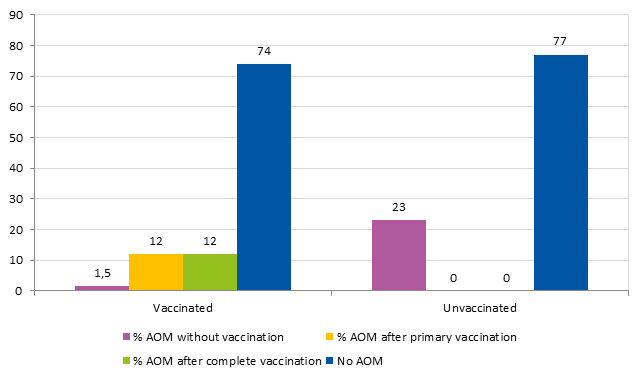 |

Attendance to childcare centres was associated with a higher incidence of AOM: 36.8% (25/68) of children who attended childcare versus 20.6% (21/102) of children who did not (P = .02; relative risk [RR]: 1.78; 95 CI: 1.09 to 2.92). We could not obtain data on childcare attendance in 24 cases. Table 2 shows the cumulative incidence of AOM at different ages and its association with childcare attendance or lack thereof prior to onset of AOM. The age at the first episode of AOM was significantly younger (P = .031) in children enrolled in childcare before the onset (median: 12 months; interquartile range: 6) compared to those that had not been in childcare (median: 18 months; interquartile range: 9).
| Table 2. Cumulative incidence of otitis at different ages by attendance to childcare centre prior to onset of otitis | ||||
|---|---|---|---|---|
| Age group | AOM in children in childcare prior to onset/total (%) | AOM in children not in childcare prior to onset/total (%) | % children who attended childcare | P |
| < 6 months | 0/3 (0.0%) | 3/167 (1.8%) | 1.8% | P = 1 |
| < 12 months | 5/24 (20.8%) | 16/146 (11.0%) | 14.1% | P = .031 |
| < 18 months | 18/49 (36.7%) | 21/121 (17.4%) | 28.8% | P = .006 |
| < 24 months | 22/57 (38.6%) | 22/113 (19.5%) | 33.5% | P = .007 |

Fourteen children (29%) had recurrent AOM: six had 2 episodes, seven had 3 episodes and one had 4 episodes. Of these children, 10 did not attend childcare and 10 were vaccinated, and only 1 among the vaccinated had recurrent AOM before vaccination.
There were 6 cases of pneumonia, 2 of them diagnosed before vaccination, 2 after primary vaccination and 2 after completion of vaccination. Three of these six children attended childcare centres. The differences were not statistically significant.
DISCUSSION
After funding was withdrawn for the PCV-13 in the CM as part of the budget cuts of 2012, there was a significant reduction in the vaccination coverage of patients in the Entrevías PCC, which dropped from 98% to 64.9% (95 CI: 57.3 to 71.4), while the rate in the CM overall dropped to 77.1%.5
We did not find an increase in pneumococcal disease. One possible explanation is the persistence of the herd immunity induced by this vaccine, which had only recently lost funding. The results may have been different if the lack of public funding had lasted longer.
Studies conducted in Kenya and Massachusetts9 concluded that herd immunity can be achieved when the vaccination coverage in children aged less than 2 years is of at least 65%. Some authors3 suggest that the vaccine may offer some degree of indirect protection with coverage rates as low as 40%.
An essential factor in achieving herd immunity is the reduction in the prevalence of nasopharyngeal carriage. Vaccination with PCV-13 reduces colonization of the nasopharynx by serotypes 1, 6A, 7F and 19A by approximately 50% compared to the PCV-7, but does not change the prevalence of carriage of serotype 3, as the PCV-13 does not achieve higher antibody titres. The greatest reduction in carriage has been found in serotype 19A, which may be due to the outstanding effectiveness exhibited by the PCV-13 against this serotype in the CM. The ecological niche created by the disappearance of these serotypes from the nasopharynx is occupied by other non-PVC-13 serotypes, although they seem to be less invasive than the vaccine serotypes.3 The use of PCV-13 reduces nasopharyngeal colonization not only in the vaccinated subpopulation but also in unvaccinated children and adults.3
Childhood immunisation indirectly protects groups of unvaccinated individuals through a reduction in the circulation of vaccine serotypes. There is evidence that prevention of pneumococcal invasive disease in adults through herd immunity is a beneficial and very cost-effective result of vaccination.10
Given the low incidence of IPD (7.7 cases per 100 000 inhabitants aged < 15 years),11 we expected not to find any cases in our study. In 2007, the CM decided to start an epidemiologic surveillance system and published its results in the Heracles study. The findings of this study6,11 and similar works2,12 evince a significant reduction in the cumulative incidence of IPD caused by vaccine serotypes in children of all ages. In the region of Madrid, despite the discontinuation of routine vaccination with PCV-13, the reduction in the incidence of IPD has persisted. With a vaccination coverage estimated at around 70%, the reduction in the incidence of IPD by all serotypes and by vaccine serotypes has been of 68% and 84%, respectively, compared to the period preceding the introduction of the vaccine.3 On the other hand, the effectiveness of the vaccine against non-invasive disease is difficult to assess, and there are fewer studies on the subject.
The main limitation of our study in assessing non-invasive pneumococcal disease is that microbial culture is not usually performed in these patients, even if they are admitted to hospital. Thus, our assessment of the differences in the incidence of AOM and pneumonia between vaccinated and unvaccinated children is limited by the uncertainty regarding the aetiology of the disease.
To reduce the risk of bias due to misdiagnosis, and since the diagnostic criteria applied to these cases were clinical, we applied the criteria established by consensus by the Asociación Española de Pediatría for the diagnosis of otitis7 and pneumonia.8 We collected the clinical data associated to these diagnoses in the health records, and only considered cases in which the diagnosis was supported by documented clinical manifestations.
The fact that we did not find a significant difference in the incidence of AOM between vaccinated an unvaccinated children, despite the unvaccinated cohort being older (longer duration of exposure), could reflect the modest protection (of between 0-9% according to randomised controlled trials and 17-23% to non-randomised trials) conferred by conjugated vaccines against this disease in low-risk patients, which is probably due to vaccines including a limited number of serotypes, as suggested by other authors.13
In 2014,Fortanier et al14 performed a meta-analysis of 11 randomised controlled trials that included a total of 48 426 children aged up to 7 years vaccinated with PCV-7, and reached similar conclusions.
The additional serotypes included in the PCV-13 (especially 19A) provide increased protection against AOM. In the United States, the proportion of vaccine serotype isolates from middle ear samples dropped from 50% of the total in 2011 to 29% in 2013, with the proportion of serotype 19A isolates dropping from 34% to 10%.15
Other studies report more favourable data. In Israel, the use of PCV-7 followed by PCV-13 achieved a reduction of 77% in the incidence of AOM caused by vaccine serotypes and of 60% in the incidence of AOM of any aetiology.16
Long-term studies17 have found a decrease in the incidence of chronic and complicated otitis media with the use of the PCV-13, as well as a reduction in ventilation tube insertion. The authors of this review acknowledge that there was variability among the included studies, partly due to differences in their assessment criteria, so that its results should be interpreted with caution.
Our results showed a lower incidence of pneumonia in vaccinated children compared to unvaccinated children, but we could not reach firm conclusions due to the small sample size and number of cases (4 cases in unvaccinated or partially vaccinated children versus 2 cases in completely vaccinated children). When it comes to the incidence of pneumonia with radiological evidence of consolidation, the effectiveness of the PCV-13 ranges between 15% and 40%,3 with vaccination achieving a reduction of 27% in hospital admissions and 8% in emergency department visits due to pneumonia according to a study conducted in the United States two years after the introduction of the PCV-13.18
While it is possible that we did not find differences in the incidence of non-invasive pneumococcal disease due to the sample size, which we had initially calculated to assess differences in vaccination coverage, we should keep in mind that herd immunity could be the reason that there were no differences in the incidence of AOM or pneumonia. The results of other studies recently carried out in the CM support this hypothesis.11
Similarly, we collected data on gestational age at birth to assess whether preterm birth could be a risk factor for the development of pneumococcal disease, but we did not find differences in the incidence of AOM or pneumonia when we compared children born before and after 37 weeks’ gestation.
In our study, we found a higher incidence of AOM in children who attended childcare centres, which was consistent with the findings of previous studies.19 Pneumococcus is transmitted by direct person-to-person contact after prolonged physical contact. There is evidence that crowding (in prisons, camping facilities, shelters…) is associated with outbreaks,4 and attendance to childcare centres with an increased risk of AOM and AOM with effusion.19
The removal of funding for the PCV-13 in the CM led to a decrease in vaccination coverage in an area with incomes below the regional average. Whereas these data do not seem to be clinically relevant, they must be taken into consideration by governing bodies when making decisions regarding vaccine funding.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article.
ABBREVIATIONS
AOM: acute otitis media • BMDS: Basic Minimum Data Set for hospital discharge and outpatient surgery • CI: confidence interval • CM: Community of Madrid • IPD: invasive pneumococcal disease • PCC: primary care centre • PCV: pneumococcal conjugate vaccine • PCV-13: 13-valent pneumococcal conjugate vaccine • PCV-7: 7-valent pneumococcal conjugate vaccine.
REFERENCES
- Van Hoek AJ, Sheppard CL, Andrews NJ, Waight PA, Slack MPE, Harrison TG, et al. Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine. 2014;32:4349-55.
- Zangeneh TT, Baracco G, Al-Tawfiq JA. Impact of conjugate pneumococcal vaccines on the changing epidemiology of pneumococcal infections. Expert Rev Vaccines. 2011;10:345-53.
- Comité Asesor de Vacunas de la AEP. Neumococo. In: Manual de vacunas en línea de la AEP [online] [accessed 24/10/2017]. Available at http://vacunasaep.org-documentos/manual/cap-31
- Enfermedad invasora por Streptococcus pneumoniae. In: Servicio Madrileño de Salud [online] [accessed 24/10/2017]. Available at: https://goo.gl/KwN2us
- Enfermedad neumocócica invasora en la Comunidad de Madrid. Año 2014. Bol Epidemiol Comunidad de Madrid. 2015;21:5-55.
- Picazo J, Ruiz-Contreras J, Casado-Flores J, Giangaspro E, García-de-Miguel MJ, Hernández-Sampelayo T, et al. Impact of introduction of conjugate vaccines in the vaccination schedule on the incidence of pediatric invasive pneumococcal disease requiring hospitalization in Madrid 2007 to 2011. Pediatr Infect Dis J. 2013;32:656-61.
- Del Castillo Martín F, Baquero Ortigao F, Calle Cabrera T, López Robles M, V Ruiz Canela J, Alfayate Migueleza S, et al. Documento de consenso sobre etiología, diagnóstico y tratamiento de la otitis media aguda. An Pediatr (Barc). 2012;77:345.e1-e8.
- Andrés Martín A, Moreno Pérez D, Alfayete Miguélez S, Couceiro Gianzo J A, García García M L, Korta Muruac J, et al. Etiología y diagnóstico de la neumonía adquirida en la comunidad y sus formas complicadas. An Pediatr (Barc). 2012;76:162.e1-e18.
- Klugman KP. Herd protection induced by pneumococcal conjugate vaccine. Lancet Glob Health. 2014;2:e365-66.
- Loo J, Conklin L, Fleming-Dutra KE, Knoll N, Park DE, Kirk J. Systematic review of the indirect effect of pneumococcal conjugate vaccine dosing schedules on pneumococcal disease and colonization. Pediatr Infect Dis J. 2014;33:161-71.
- Picazo J, Ruiz-Contreras J, Casado-Flores J, Negreira S, García-de-Miguel MJ, Hernández-Sampelayo T, et al. Expansion of serotype coverage in the universal pediatric vaccination calendar: short-term effects on age- and serotype-dependent incidence of invasive pneumococcal clinical presentations in Madrid, Spain. Clin Vaccine Immunol. 2013;20:1524-30.
- Waight PA, Andrews NJ, Ladhani NJ, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15:629-33.
- Taylor S, Marcjisio P, Vergison A, Harriague J, Hausdorff WP, Haggard M. Impact of pneumococcal conjugate vaccination on otitis media: a systematic review. Clin Infect Dis J. 2012;54:1765-73.
- Fortanier AC, Venekamp RP, Boonacker CWB, Hak E, Schilder AGM, Sanders EAM, et al. Pneumococcal conjugate vaccines for preventing otitis media (review). Cochrane Database Syst Rev. 2014:3-17.
- Cohen R, Levy C, Bingen E, Koskas M, Nave I, Varon E. Impact of the 13-valent pneumococcal conjugate vaccine on pneumococcal nasopharyngeal carriage in children with acute otitis media. Pediatr Infect Dis J. 2012;31:297-301.
- Ben-Shimol S, Givon-Lavi N, Leibovitz E, Raiz R, Greenberg D, Dagan R. Near-elimination of otitis media caused by 13-valent pneumococcal conjugate vaccine (PCV) serotypes in Southern Israel shortly after sequential introduction of 7-valent/13-valent vaccine. Clin Infect Dis. 2014;59:1724-32.
- Dagan R, Pelton S, Bakaletz L, Cohen R. Prevention of early episodes of otitis media by pneumococcal vaccines might reduce progression to complex disease. Lancet Infect Dis. 2016;16:480-92.
- Griffin MR, Mitchel E, Moore MR, Whitney CG, Grijalva CG. Declines in pneumonia hospitalizations of children aged <2 years associated with the use of pneumococcal conjugated vaccines. MMWR Morb Mortal Wkly Rep. 2014;63:995-8.
- Ochoa Sangrador C. ¿Cuánto aumenta el riesgo de otitis media aguda la asistencia a guardería? Evid Pediatr. 2007;3:108.
Comments
This article has no comments yet.





