Vol. 25 - Num. 97
Original Papers
COVID-19 in pediatrics: clinical and microbiological description of patients admitted to a tertiary hospital
Celia Rasero Bellmunta, M.ª Carmen Vicent Castellóa, Amelia Herrero Galianaa, Javier González de Diosb
aServicio de Pediatría. Hospital General Universitario Dr. Balmis. Alicante. España.
bServicio de Pediatría. Hospital General Universitario Dr. Balmis. Alicante. Facultad de Medicina. Universidad Miguel Hernández. Alicante. Instituto de Investigación Sanitaria y Biomédica de Alicante (ISABIAL). España.
Correspondence: C Rasero. E-mail: celiarasero68@gmail.com
Reference of this article: Rasero Bellmunt C, Vicent Castelló MC, Herrero Galiana A, González de Dios J. COVID-19 in pediatrics: clinical and microbiological description of patients admitted to a tertiary hospital . Rev Pediatr Aten Primaria. 2023;25:21-30.
Published in Internet: 14-03-2023 - Visits: 14404
Abstract
Introduction: since the beginning of the SARS-CoV-2 pandemic, one of the main questions that has been asked is what role children play in the control and management of the pandemic and how it has affected them. There is much literature on the symptoms and complications that this population may have, but little on the clinical course of the infection in children admitted to tertiary hospitals and its impact on health care.
Material and methods: the clinical histories of children admitted to the Hospital General Doctor Balmis (Alicante, Spain) from January 2020 to July 2022 were analyzed descriptively. At the same time, microbiological data on SARS-CoV-2, variants and lineages were analyzed from August 2021 to August 2022.
Results: a total of 114 children admitted were analyzed, most of whom were younger than 12 months and from Spain. Admissions were distributed chronologically following a 'wave' pattern, the most frequent reason being the finding of SARS-CoV-2 virus in the tests performed. The most common treatment received during admission was oral antibiotics. Most of the children had no comorbidities and did not develop complications.
Discussion: infants seem to be more vulnerable to SARS-CoV-2 infection, and clinical manifestations in this age group are more likely to lead to admission. The development of complications, need for oxygen therapy, mechanical ventilation and admission to the ICU is minimal in the pediatric population. The management of infection differs substantially from that of adults, which corresponds to less aggressive treatment.
Keywords
● Diagnosis ● Pediatrics ● SARS-CoV-2 ● Treatment ● VariantsINTRODUCTION
On December 8, 2019, the first case of infection by SARS-CoV-2 was detected in Wuhan, a city located in southern China.1 Gradually, more cases were identified and evidence emerged that they had in common the exposure to a market of seafood, fish and live animals. However, the causative agent was not identified until January 7, 2020. In Spain, the first case was detected on January 31, 2021 and on March 11 of the same year, the World Health Organization declared the novel coronavirus outbreak a global pandemic. Three days later, on March 14, the Spanish government declared the state of alarm. One month later, 1 996 681 cases had been reported worldwide (177 633 in Spain), along with 127 601 deaths (18 579 in Spain). As the time of this writing (November 2022), es de 548 935 393 cases (13 529 643 in Spain) and 6 350 765 deaths (115 239 in Spain). In the context of this pandemic, 2 key questions emerged in relation to paediatric care: how did it affect the paediatric population? and, were children a key component in the control and management of the pandemic?
Children typically become infected through exposure in the home, as adults are usually the index case. Children seem to shed virus in nasopharyngeal secretions with viral loads that are comparable to or greater than those observed in adults. There is also viral shedding through the stools for several weeks after diagnosis, which could be a challenge for the control of transmission.2,3
In children, the infection tends to be asymptomatic (in around 15-42% of the total) or cause mild symptoms. The exact mechanisms by which the disease course is milder in children compared to adults remain unknown, and several hypotheses have been proposed: a less intense immune response to the virus with a decreased release of cytokines in children, interactions between the virus and the respiratory tract of children resulting in a lower viral load; differences in the behaviour of the angiotensin-converting enzyme 2 (ACE2) receptor in different organs and systems in children compared to adults, an earlier mucosal response in children, etc.
Based on the clinical presentation, children can be divided into 2 main age groups: younger than 12 months and older than 12 months. In children aged less than 12 months, the most frequent symptoms are food refusal, fever without focus, bronchiolitis and apnoea. In children aged more than 12 months, the symptoms are similar to those of adults: respiratory manifestations (cough, nasal discharge, dyspnoea), gastrointestinal manifestations (diarrhoea, vomiting, abdominal pain), neurologic manifestations (headache, acute encephalopathy, seizures, weakness), cutaneous symptoms (maculopapular or vesicular rash, urticaria, transient livedo reticularis, acral peeling lesions), cardiovascular manifestations (heart failure, arrhythmia, myocarditis, pericarditis, cardiogenic shock, pulmonary embolism), renal manifestations (acute kidney injury).4
Complications are also rare in children. However, they must be known so that they can be detected early and the associated sequelae prevented. Multisystem inflammatory syndrome in children (MIS-C) is a rare disease that may develop as a result of SARS-CoV-2 infection; it is characterised by persistent fever, hypotension, gastrointestinal symptoms, a skin rash, myocarditis and laboratory test results consistent with increased inflammation. What has come to be known as “long COVID” consists in the persistence of symptoms 4 or more weeks after infection, and the most frequent symptoms are fatigue, weakness, headache, sleep disturbances, muscle and joint pain, respiratory problems, arrhythmia and changes in smell or taste.
There is a substantial body of evidence on the subject of SARS-CoV-2 infection in the paediatric population. However, little has been published regarding the course of disease in children admitted to tertiary care hospitals and the associated health care burden. The primary objective of our study was to describe the clinical and microbiological characteristics of SARS-CoV-2 infection in the paediatric patients admitted to the Hospital General Universitario Doctor Balmis of Alicante, Spain, during the pandemic (January 2020 to July 2022), and the secondary objective to assess the clinical impact of COVID-19 in the department of paediatrics of our hospital.
MATERIAL AND METHODS
We obtained a list of all paediatric patients (age ≤14 years) admitted with a diagnosis of SARS-CoV-2 infection from the beginning of the pandemic (January 2020) to July 2022 from the intake department of the hospital.
We collected data on patient age and nationality (Spanish or other), dates of admission and discharge, primary diagnosis at admission, presenting symptoms and symptoms during hospital stay (fever, cough, nasal discharge, dyspnoea, abdominal pain, diarrhoea, nausea, vomiting, food refusal, myalgia, headache…), comorbidities, need of admission to intensive care unit (ICU), oxygen therapy and mechanical ventilation, complications and treatment.
Through the department of microbiology of the hospital (the reference laboratory of the province of Alicante for sequencing samples of COVID-19 patients), we obtained access to the sequencing results for samples obtained from paediatric patients, inpatient or outpatient, over a 1-year period (August 2021 to August 2022).
The descriptive analysis of the data was conducted with the software R Commander.
RESULTS
We identified a total of 161 patients admitted with a diagnosis of SARS-CoV-2 infection in the 31 months from the beginning of the pandemic comprehended in the study. We excluded hospitalized patients in whom a review of the health records revealed that the reason for admission was not COVID-19 and in whom the detection of SARS-CoV-2 was a chance finding in the tests carried out at admission (eg, preoperative evaluation). After this selection process, the sample included a total of 114 paediatric patients with COVID-19.
Study variables
- Age. Forty-nine percent of the children admitted to our department were aged less than 12 months (Table 1). In this subset, 60% were aged 1 to 2 months.
Table 1. Age distribution of the 114 children admitted due to COVID-19 through July 2022 Age Frequency Percentage <12 months 56 49% <1 month 5 8% 1 month 21 37% 2 months 13 23% 3 months 6 11% 4 months 2 4% 5 months 2 4% 6 months 4 7% 7 months 0 0% 8 months 1 2% 9 months 2 4% 10 months 0 0% 11 months 0 0% 1 year 10 9% 2 years 9 8% 3 years 8 7% 4 years 8 7% 5 years 3 3% 6 years 3 2% 8 years 2 2% 9 years 3 2% 10 years 3 3% 11 years 2 2% 12 years 2 2% 13 years 5 4% 
- Nationality. Seventy-four percent of patients had been born in Spain and the rest were from various foreign countries (Argelia, Colombia, Argentina, Ecuador, etc.).
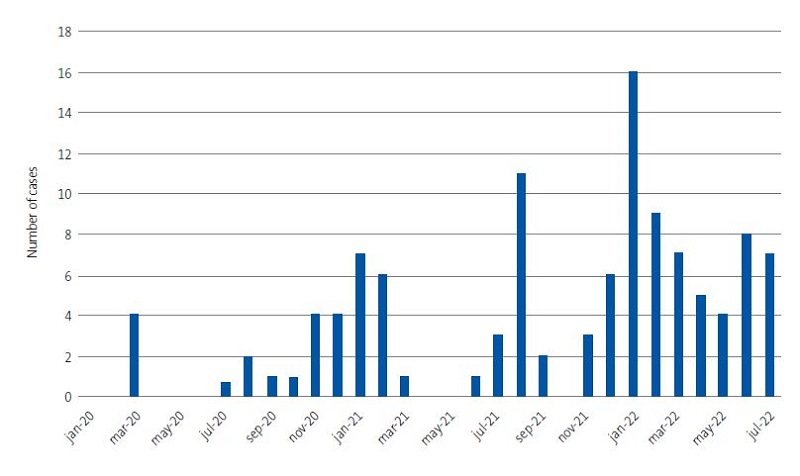
- Dates and frequency of admissions. The chronological distribution of hospital admissions exhibited a wave pattern coinciding with the waves observed in the general population (Figure 1). The number of admissions peaked in January 2022, with a total of 14 admissions, and there were no admissions in January and May 2020 and in May 2021.
- Reason for admission. The reason for admission in 68% of the patients was the detection of SARS-CoV-2 in the context of the diagnostic workup, as can be seen in Table 2. Other diagnoses recorded at admission were bronchospasm (10%), febrile illness (6%), acute gastroenteritis (5%), pneumonia (5%) and MIS-C (4%).
- Manifestations of SARS-CoV-2 infection. The most frequent symptom (at admission and during the hospital stay) was fever, present in 72% of the children, followed by cough (54%) and, in third place, rhinorrhoea (52%). Less frequent symptoms included food refusal, diarrhoea, shortness of breath, nausea and vomiting, abdominal pain, myalgia and headache (Table 3).
Table 3. Symptoms of infection by SARS-CoV-2 in 114 children admitted due to COVID-19 through July 2022 Symptoms Frequency Percentage Fever 83 72% Cough 61 54% Rhinorrhoea 60 52% Food refusal 43 38% Diarrhoea 27 24% Shortness of breath 27 24% Nausea/vomiting 23 20% Abdominal pain 9 8% Myalgia 7 6% Headache 4 4% 
- Comorbidities. Only 23% of the hospitalised patients had comorbidities (Table 4). The most frequent comorbidities were diseases associated with immunosuppression, such as leukaemia, lymphoma, primary immunodeficiency or immunosuppressive therapy post transplantation. Other observed comorbidities were neurologic (Rett syndrome, West syndrome, central nervous system malformations), respiratory (childhood asthma), renal (solitary kidney), psychiatric (depression under pharmacological treatment ) or genetic (L1 syndrome, Down syndrome).
Table 4. Distribution of comorbidities, overall and by type, in 114 children admitted due to COVID-19 through July 2022 Comorbidity Frequency Percentage No 89 77% Yes 25 23% Type of comorbidity Immunosuppression 12 46% Neurologic 5 19% Respiratory 4 15% Infectious 1 4% Renal 1 4% Psychiatric 1 4% Other* 2 8% *Including renal comorbidities (solitary kidney), psychiatric comorbidities (depression currently in treatment), genetic disorders (L1 syndrome , Down syndrome). 
- Need of oxygen therapy, mechanical ventilation and admission to the paediatric ICU. Most patients did not require admission to the ICU, oxygen therapy or mechanical ventilation (88.9 and 96%, respectively). Of the 14 patients who required admission to the ICU, 5 had concomitant comorbidities (childhood asthma, Rett syndrome, central nervous system malformations, Down syndrome and severe malaria), and 6 had developed complications (1, mitral insufficiency; 3, severe dehydration with acidosis; 2, respiratory failure). The most frequently used oxygen therapy modality was supplemental oxygen with nasal prongs (9 patients), followed in frequency by high-flow oxygen therapy (5). Of the 5 children who required mechanical ventilation, 4 required invasive ventilation and all required admission to the ICU; only 1 received non-invasive mechanical ventilation.
- As can be seen in Table 5, the most frequent complications involved the respiratory tract (45%), such as pneumonia (in most cases) and respiratory failure. Other types of complications had a fairly even distribution: haematological (neutropaenia, hyperbilirubinaemia), metabolic (dehydration, hypopotassaemia), otorhinolaryngological (acute otitis media, sinusitis), cardiovascular (mitral insufficiency, heart failure).
Table 5. Distribution of complications, overall and by type, in 114 children admitted due to COVID-19 through July 2022 Complications Frequency Percentage No 96 84% Yes 18 16% Type of complication Respiratory 8 44.5% Metabolic 3 16.7% Haematological 2 11.1% Otorhinolaryngological 2 11.1% Cardiovascular 2 11.1% Other* 1 5.5% *Includes onset of MIS-C. 
- Treatment. Forty-two percent of patients received pharmacological treatment (Table 6), most frequently oral antibiotherapy (azithromycin, amoxicillin-clavulanic acid, ceftriaxone), followed by oral steroid therapy and bronchodilators (nebulised and inhaled salbutamol and ipratropium bromide).
Table 6. Treatments received by the 114 children admitted due to COVID-19 through July 2022 Treatment Frequency Percentage Oral antibiotherapy 25 29% Oral steroid therapy 13 15% Bronchodilator drugs 11 13% Intravenous steroid therapy 7 8% IVIG 6 7% LMWH 5 6% Remdesivir 3 4% ASA 3 4% Nebulised adrenaline 3 4% Intravenous antibiotherapy 2 2% ASA: acetylsalicylic acid; LMWH: low molecular weight heparin; IVIG: intravenous immunoglobulin. 
SARS-CoV-2 variants and lineages identified in the paediatric population of the province of Alicante between August 2021 and August 2022
The department of microbiology of our hospital provided data for 197 nasopharyngeal samples from paediatric patients (≤16 years) subjected to sequencing, including the date of sample collection, age and sex of the patient, setting of sample collection, variant and lineage.
- Age and sex. Fourteen percent of the sequenced samples were from infants aged less than 1 year, with a more uniform percent distribution for the rest of the age groups (3-8%). The distribution by sex was also uniform (49% male and 51% female).
- Origin of samples. Most sequencing samples had been collected in primary care centres in the province of Alicante (56%), and 26% were collected in the inpatient wards of the Hospital General Universitario Dr. Balmis, 8% in the emergency department of the same hospital and the remaining 10% from educational facilities.
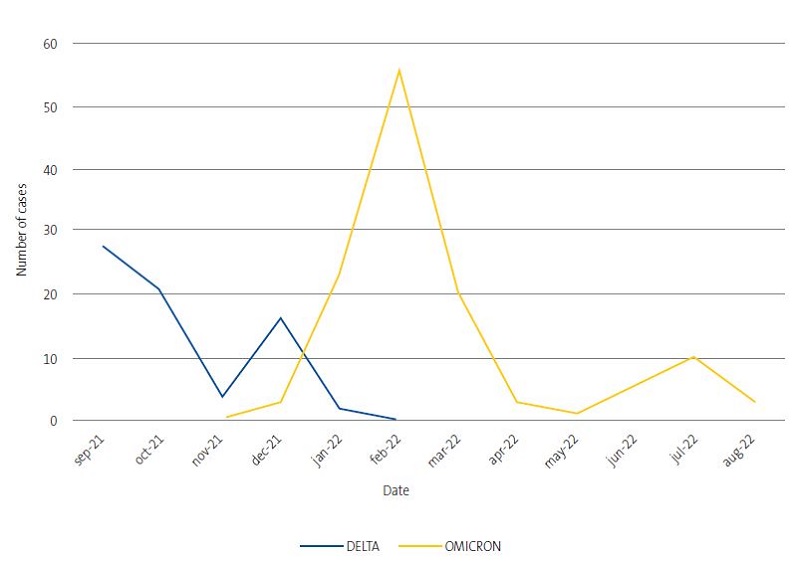
- SARS-CoV-2 variants. In our sample, the predominant variant was omicron (63% of sequenced nasopharyngeal samples). The most frequent lineage was BA.1 (20%), followed by BA.2 (18%); BA.1.1 (14%) and AY.4 (13.7%). Figure 2 present the temporal trends in the prevalence of SARS-CoV-2 variants. In the last months of 2022, omicron was the most prevalent variant.
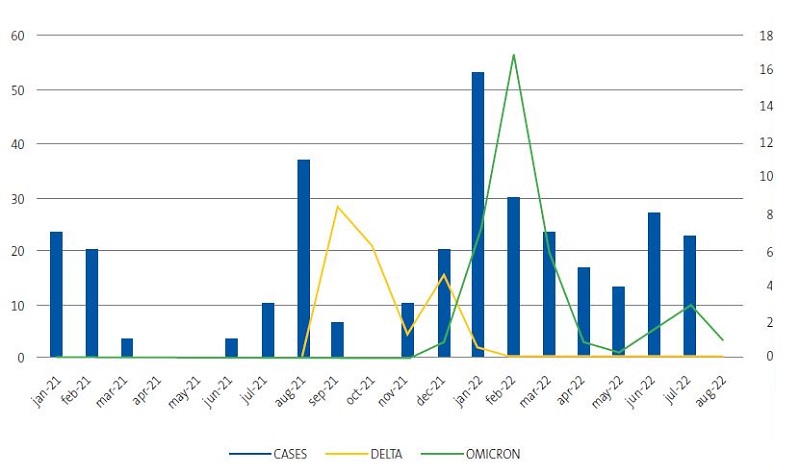
- If we compare the temporal distribution of admissions to our department (Figure 1) to the temporal distribution of SARS-CoV-2 variants in the paediatric population of the province of Alicante, we obtain Figure 3. This figure shows that in the wake of the summer 2021 and winter 2022 pandemic waves, the number of sequenced samples increased in parallel to the number of admissions, with some lag in the number of sequenced samples.
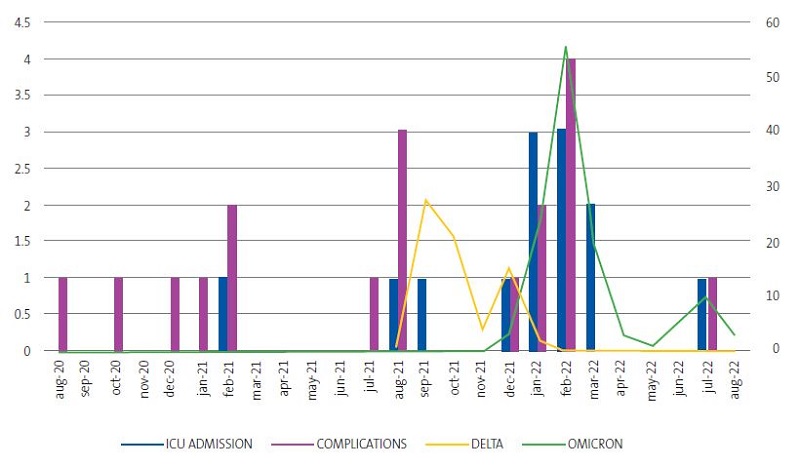
Last of all, we analysed the temporal distribution of the variables of interest and of severe cases (severity defined as admission to the ICU or development of complications), represented in Figure 4. We found that the omicron variant in the winter months of 2022 was associated with an increase in ICU admissions and in the incidence of complications compared to the delta variant.
DISCUSSION
There is a substantial body of evidence on the subject of infection by SARS-CoV-2 in children, but little has been published on the temporal trends in SARS-CoV-2 infection in paediatric patients admitted to tertiary care hospitals during the pandemic. One source of information on the subject is the document developed by the Committee/Group on Evidence-Based Paediatrics of the AEP and AEPap.4 According to this document, infants seem to be more vulnerable to infection, with which our findings were consistent, as infants were the age group with the highest rate of admission. In this regard, it is worth noting that children under 12 months are more likely to be admitted, as the clinical manifestations of SARS-CoV-2 infection (fever of unknown aetiology, food refusal, apnoea) are established criteria for hospital admission.5 Infants aged 1 to 2 months were admitted most frequently, as age 1 to 3 months (combined with the findings of the clinical assessment) is also one of the criteria taken into account for admission. However, it is still unclear whether infection by SARS-CoV-2 causes severe respiratory disease, such as acute bronchiolitis, in this age group. In fact, during the pandemic there was a drastic decrease in the incidence of bronchiolitis and in paediatric ICU admissions related to infection by respiratory syncytial virus (RSV), among others,6 probably due to the established isolation measures, which curbed the transmission of all types of viral infection.
According to the literature,4 the most frequent symptom of SARS-CoV-2 infection in children is fever (55.8%), followed by cough (42.2%); these percentages are similar to those observed in the paediatric patients admitted to our hospital. The rest of the symptoms appeared in proportions that differed more from previous publications. This could be due to the small size of our sample, on account to which the variations observed in the less frequent symptoms may be greater compared to studies with larger samples..
Another aspect worth highlighting is the different approach to the management of the infection in adult versus paediatric patients. In our sample, we found that the drugs that have been the cornerstone of treatment in adult patients, such as intravenous steroids, low molecular weight heparin and remdesivir, were not the ones used most frequently in children (only in 9, 6 y 4%, respectively). In our sample, oral antibiotherapy was the most commonly prescribed treatment (29%), compared to its use in 55-85% of hospitalised adult patients, which was probably due to the greater severity of SARS-CoV-2 (with a higher proportion of bacterial superinfection) in adults.7 This reflects one of the key differences in management, as in the paediatric age group the therapeutic approach tends to be less aggressive and there is less data demonstrating the effectiveness of the drugs used in adults to manage COVID-19.5 In addition, the incidence of complications is generally lower in the paediatric population, which would explain the less frequent need of aggressive treatments. In our study, we defined severity chiefly based on the need of ICU admission, oxygen therapy and mechanical ventilation, which were only required in 12, 12 and 4% of patients, respectively. These figures were consistent with those reported in the previous literature,4,8 according to which approximately 11.6% of children hospitalised due to COVID-19 required admission to the ICU and 4% required invasive mechanical ventilation.
Concerning the different SARS-CoV-2 variants, in our study we found that, following the waves of summer 2021 and winter 2022, the number of sequenced samples increased at the same time as the number of cases requiring admission (with some lag in the frequency of sequencing). The most plausible explanation is that the clinical evidence that the number of admissions was increasing prompted the increase in the number of orders of DNA sequencing for microbiological diagnosis.
Based on the current literature,9 the delta variant seems to be associated with greater disease severity, but a lower infectivity, while the omicron variant seems to be more infective but cause milder disease. In our study, the omicron variant predominated from August 2021, with a parallel increase in the frequency of admissions to the ICU and the incidence of complications. This does not contradict the published evidence, as there is little data on the variant that predominated between January and July 2021, both included.
The findings reported in this article are subject to the limitations intrinsic to retrospective descriptive studies. First of all, the lack of randomization in sampling entails a risk of selection bias, as patients who were admitted may not have been representative of the general paediatric population (accessibility of hospital-based care, geographical distribution of the paediatric population, etc). Thus, the purpose of the study was not to establish causality, but to make a descriptive analysis. Secondly, the information obtained from hospital records may be incomplete, and some data may be subject to interobserver variation (such as the assessment of the presence or absence of dyspnoea). In addition, the criteria used to define severity (oxygen therapy, mechanical ventilation, need of admission to the ICU) have not been standardised across scientific societies, and each hospital department adapts them to its particular needs.
In conclusion, in the department of paediatrics of the Hospital General Universitario Doctor Balmis, the rate of admissions due to COVID-19 was highest in infants under 12 months, most of who were aged 1-2 months. The most frequent symptom was fever, followed by cough and rhinorrhoea. The most frequently detected comorbidity was immunosuppression. Most of the patients did not require supplemental oxygen, mechanical ventilation or admission to the ICU. The omicron variant was the SARS-CoV-2 variant that predominated in recent months in the paediatric population of the province of Alicante. Concurrently, although we do not know whether there is a causal relationship on account of the aforementioned lack of data, there has been an increase in the incidence of complications and ICU admission in the paediatric population. This could be due to a temporal bias due to differences in the periods of data collection for children admitted to our department (from the start of the pandemic in January 2020) and for microbiological testing (which started in August 2021).
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article.
AUTHORSHIP
All authors contributed equally to the manuscript.
ABBREVIATIONS
ICU: intensive care unit · MIS-C: multisystem inflammatory syndrome in children · RSV: respiratory syncytial virus.
REFERENCES
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-20.
- Lu X, Zhang l, Du H, et al. SARS-CoV-2 Infection in Children. N Engl J Med. 2020;382:1663-5.
- Deville JG, Song E, Ouellette CP. COVID-19: Clinical manifestations and diagnosis in children. In: UpToDate; 2022 Aug [online] [accessed 31/08/2022]. Available at www.uptodate.com/contents/covid-19-clinical-manifestations-and-diagnosis-in-children#H1359939953
- Comité/Grupo de Pediatría Basada en la Evidencia de la AEP y AEPap. COVID-19 en Pediatría: valoración crítica de la evidencia. In: AEPap [online] [accessed 15/11/2022]. Available at www.aepap.org/grupos/grupo-de-pediatria-basada-en-la-evidencia/biblioteca/covid-19-en-pediatria-valoracion-critica-de-la-evidencia
- Alteri C, Scutari R, Costabile V, Colagrossi l, Yu La Rosa K, Agolini E, et al. Epidemiological characterization of SARS-CoV-2 variants in children over the four COVID-19 waves and correlation with clinical presentation. Sci Rep. 2022;12:10194.
- SEIP, SEUP, SECIP. Documento de manejo clínico del paciente pediátrico con infección por SARS-CoV-2. Extracto del Documento de Manejo Clínico del Ministerio de Sanidad [online] [accessed 15/11/2022]. Available at www.aeped.es/sites/default/files/b26-11-_aep-seip-secip-seup._documento_de_manejo_clinico_del_paciente_pediatrico.pdf
- Aguilera Alonso D, Eplaza C, Sanz Santaeufemia FJ, Grasa C, Villanueva Medina S, Melendo S, et al. Antibiotic Prescribing in Children Hospitalized With COVID-19 and Multisystem Inflammatory Syndrome in Spain: Prevalence, Trends, and Associated Factors. J Pediatr Infect Dis Soc. 2022;11:225-8.
- Tagarro A, Cobos Carrascosa E, Villaverde S, Sanz Santaeufemia FJ, Grasa C, Soriano Arandes A, et al. Clinical spectrum of COVID-19 and risk factors associated with severity in Spanish children. Eur J Pediatr. 2022;181:1105-15.
- Andina Martínez D, Alonso Cadenas JA, Cobos Carrascosa E, Bodegas E, Oltra Benavent M, Plazaola A, et al. SARS-CoV-2 acute bronchiolitis in hospitalized children: Neither frequent nor more severe. Pediatr Pulmonol. 2022;57:57-65.