Vol. 23 - Num. 91
Original Papers
Clinical features and diagnostic difficulties from a prospective study of community-acquired pneumonia in children
Mercedes Cemeli Canoa, M.ª Esperanza Sáez de Adana Péreza, Juan José Lasarte Velillasb, M.ª Isabel Moneo Hernándezc, Pilar Samper Villagrasad, César García Verae
aPediatra. CS Valdespartera-Montecanal. Zaragoza. España.
bPediatra. CS Torre Ramona. Zaragoza. España.
cPediatra. CS Las Fuentes Norte. Zaragoza. España.
dDepartamento de Pediatría, Radiología y Medicina Física. Universidad de Zaragoza. Zaragoza. España.
ePediatra. CS José Ramon Muñoz Fernández. Zaragoza. España.
Correspondence: M Cemeli. E-mail: cano.mcemeli@salud.aragon.es
Reference of this article: Cemeli Cano M, Sáez de Adana Pérez ME, Lasarte Velillas JJ, Moneo Hernández MI, Samper Villagrasa P, García Vera C. Clinical features and diagnostic difficulties from a prospective study of community-acquired pneumonia in children. Rev Pediatr Aten Primaria. 2021;23:273-83.
Published in Internet: 13-09-2021 - Visits: 14330
Abstract
Introduction: pneumonia is one of the main causes of morbidity and mortality in children. Its management at the paediatric primary care level is not yet solidly established.
Material and methods: we conducted a prospective study in children aged 1 month to 14 years included in the paediatric caseloads of 9 paediatric primary care centres. The aetiological diagnosis was made by means of serology tests and viral testing in nasopharyngeal aspirate samples. We analysed the association of different variables with the aetiology of pneumonia (viral, atypical bacterial and typical bacterial).
Results: the study included 92 patients. The mean age (47.58 months) was significantly higher in cases of atypical pneumonia (74.2 ± 42.2) compared to viral pneumonia (36.1 ± 44.5; p <0.0001) and probable typical pneumonia (32.6 ± 21.1; p <0.0001). The distribution by aetiology was 33.7% (95 CI: 24.9 to 43.8) probable pneumococcal, 30.4% (95 CI: 22.0 to 40.5) atypical and 21.7% (95 CI: 14.5 to 31.2) viral. Atypical pneumonia was relatively frequent in children under 5 years (17.1%). Fever (89.1%) and cough (68.4%) were the most frequent symptoms. The most common radiographic feature was alveolar infiltration (53.8%), with no differences between groups. C-reactive protein levels were significantly higher in viral cases (7.6 ± 9.5) compared to probable pneumococcal cases (4.9 ± 10.1) (DM: -2.7; p = 0.0490) and atypical cases (1.7 ± 1.7) (DM: -5.83; p = 0.0111). Amoxicillin was the most frequently used antibiotic (66.3%), which achieved favourable outcomes in all types of pneumonia. The frequency of hospital admission was higher in patients with viral pneumonia (50%) compared to atypical (7.4%; p = 0.0023) or probable pneumococcal pneumonia (12.90 %; p = 0.0100).
Conclusion: the epidemiology of community-acquired pneumonia in the paediatric population is changing, especially when it comes to atypical bacterial and viral causative agents. The aetiological diagnosis and optimal antibiotherapy regimen for pneumonia are goals yet to be achieved.
Keywords
● Pediatrics ● Pneumonia ● Primary careINTRODUCTION
Community-acquired pneumonia (CAP) is the most frequent individual cause of mortality worldwide. Its high annual incidence, of 30 to 40 cases per 1000 children under 5 years, and its potential severity, are an important source of anxiety and a substantial use of resources. One of the main challenges in children with CAP is to achieve an aetiological diagnosis, which is particularly difficult in the primary care (PC) setting. Identification of the aetiological agent depends on the availability of diagnostic tests, due to which the proportion of cases in which identification is achieved ranges from 40% to 85% in the recent literature.1,2
Despite the high morbidity and mortality associated with CAP, there is no universally accepted standardised diagnostic protocol.3 The evidence on the performance of chest radiography for differentiation of a bacterial versus a viral aetiology and prediction of severity in children is scarce.4-6 The uncertainty in the diagnosis of CAP in children contributes to the excessive prescription of antibiotherapy in children with viral respiratory infections and the irrational use of broad-spectrum antibiotics.4,7
It is believed that Streptococcus pneumoniae is the most frequent causative agent in bacterial pneumonia, but bacteraemia only occurs in 10% of paediatric patients, and there are no other effective non-invasive approaches to diagnosis. In addition, viruses are important pathogens in children, and may be involved in 23% to 35% of pneumonia cases in the form of coinfection.
Thus, it is difficult to differentiate bacterial and viral pneumonia or typical and atypical pneumonia based solely on clinical signs and symptoms. Consequently, it is important to be aware of regional changes in epidemiology for the purpose of revising and improving care protocols for pneumonia in paediatric practice. In our study, we analysed the aetiology and epidemiology of CAP and recent changes, and assessed the usefulness of certain diagnostic tests, such as measurement of acute phase reactants, microbiological tests and imaging, to guide the management of CAP in primary care paediatrics.
MATERIAL AND METHODS
We conducted a prospective observational and analytic study in 9 paediatrics caseloads of PC centres in the province of Zaragoza (Spain), with selection of participants by means of cluster sampling. We included patients aged 1 month to 14 years with an initial diagnosis of pneumonia based on clinical and radiographic findings in the 2017-2019 period.
Chest radiography was used as the gold standard for diagnosis of CAP, and all chest radiographs were performed and interpreted by a single department of radiology.
The case definition of pneumonia was presence in a previously healthy patient of symptoms compatible with respiratory infection (fever, cough, difficulty breathing, rhinitis) associated with an alveolar pattern (lobar or segmental consolidation with bronchogram or pleural effusion in pneumonias of probable bacterial aetiology), an interstitial pattern (bilateral diffuse perihilar infiltrates, air trapping and atelectasis in case of atypical or viral pneumonia), a mixed pattern or an undefined pattern on radiography.
The exclusion criteria were age less than 1 month or more than 14 years; diagnosis of primary or secondary immunodeficiency, cancer, lung disease (cystic fibrosis, poorly controlled asthma, pulmonary sequestration, bronchiectasis, bronchopulmonary dysplasia), encephalopathy associated with risk of bronchial aspiration; positive tuberculin or purified protein derivative test; hospitalization in the 7-14 days prior to onset of pneumonia symptoms or onset of pneumonia in the first 24 hours of the hospital stay; lack of informed consent; diagnosis of pneumonia by a clinician other than the one usually involved in the study or in a setting other than the PC clinic more than 5 days prior.
The primary outcome was the type of pneumonia diagnosed (probably pneumococcal bacterial pneumonia, atypical bacterial pneumonia, viral, mixed). The secondary variables under study were demographic (age, sex), epidemiological (personal history, including prior history of bronchitis or bronchial hyperresponsiveness, vaccination status and history of breastfeeding), clinical (maximum temperature and duration of fever, cough, respiratory rate interpreted applying the World Health Organization cut-offs for age,8 signs of respiratory distress, oxygen saturation and extrapulmonary manifestations), radiographic (pattern, presence and location of pleural effusion or other complications), laboratory-related (total white blood cell count, neutrophils, C-reactive protein [CPR], Mycoplasma pneumoniae IgM, viral testing in nasopharyngeal samples) and treatment-related (treatment, route of administration and duration).
The diagnosis of atypical pneumonia was based on serology testing for M. pneumoniae (enzyme-linked immunoassay based on the reaction of antibodies present in the sample with the antigen bound to the polystyrene surface), which allows quantification of IgM and offers a sensitivity of 81-89% in children.1,9
The diagnosis of viral pneumonia was based on viral detection in nasopharyngeal aspirate (NPA) samples by means of immunofluorescence in the reference laboratory, using a panel that included the following viruses: respiratory syncytial virus (VRS), influenza A and B, virus, parainfluenza 1, 2 and 3, metapneumovirus and adenovirus.
Lastly, cases classified as probable pneumococcal pneumonia were those that did not meet the criteria for any of the other types of pneumonia.
In the analysis of the different types of pneumonia, we grouped cases into the following categories: probable pneumococcal bacterial pneumonia, atypical bacterial pneumonia (M. pneumoniae), viral pneumonia and mixed pneumonia.
Data collection and processing adhered to Organic Law 15/99 on the Protection of Personal Data. The database of the study did not include any personal identifiable information. Each patient was assigned a code that was only known to the research team.
The study was approved by the Research Ethics Committee of the Autonomous Community of Aragon (CEICA) on February 17, 2017, file no. C.P.-C.I. PI17/0000, minute no. 02/2017.
Statistical analysis
In the descriptive analysis, we calculated measures of central tendency and dispersion for quantitative variables (mean, median and standard deviation); and absolute frequency and percentage distributions with the corresponding 95% confidence intervals (95 CI) for qualitative variables. In the inferential analysis, we assessed the associations between variables by calculating odds ratios (ORs) and differences in means (DMs) with their respective 95% confidence intervals (95 CI). We used the χ2 test with the Yates correction or the Fisher exact test and the Student t test to assess statistical significance. When comparing means, if the distribution of any variable was not normal, we used the nonparametric Mann-Whitney U test. We used univariate regression analysis to assess the association of potential aetiologies with clinical, laboratory and radiographic variables. We also fitted a multivariate logistic regression model to predict the type of pneumonia based on the values of different variables. The selection of variables to include in the model was based on the association with the type of pneumonia in the univariate analysis (with a p-value <0.10), or biological plausibility based on the previous medical literature.
In every comparison, statistical significance was defined as a p-value ≤0.05.
RESULTS
The study included a total of 92 patients, all of which completed the followup. Of all cases, 55.2% were diagnosed in PC clinics and 43.5% in the emergency department of the corresponding referral hospital.
The sex distribution was uniform (53.2% female). The age distribution was not normal, with ages ranging from 1 month to 14 years, with a mean of 47.5 ± 40.4 months and a median of 33 months (interquartile range: 21-60).
Most episodes took place in autumn (34.7%) and spring (29.3%). Probable pneumococcal pneumonia was more frequent in autumn, while viral pneumonia was more frequent in winter and spring. Atypical pneumonia was more frequent in spring and autumn.
A previous history of bronchitis was reported by 23.9% (95 CI: 16.3 to 33.5) and a history of pneumonia by 9.7% (95 CI: 5.2 to 17.1), while 60.8% of patients did not have a relevant history.
Table 1 presents the distribution of the different types of pneumonia by age group. The mean age in months was significantly greater in cases of atypical pneumonia (74.2 ± 42.2 months) compared to probable typical pneumonia (32.6 ± 21.1 months) (DM: -41.5 months; 95 CI: -59.4 to -23.6 months; p <0.001) and viral pneumonia (36.1 ± 44.5 months) (DM: 38.1 months; 95 CI: 12.3 to 63.8; p <0.001). However, we did not find a significant difference between probable typical and viral pneumonia (DM: -3.4 months; 95 CI: -25.4 to 18.4; p = 0.3472).
| Table 1. Distribution of types of pneumonia by age group (N = 92) | ||||
|---|---|---|---|---|
| Type of pneumonia | ≤24 months n (%) |
25-60 months n (%) |
>60 months n (%) |
Total n (%) |
| Suspected pneumococcal | 9 (31.0) | 20 (48.7) | 2 (9.0) | 31 (33.6) |
| Atypical bacterial | 5 (17.2) | 7 (17.0) | 16 (72.7) | 28 (30.4) |
| Viral | 11 (37.9) | 6 (14.6) | 3 (13.6) | 20 (21.7) |
| Mixed | 1 (3.4) | 3 (7.3) | 1 (4.5) | 5 (5.4) |
| Total | 29 (31.5) | 41 (46.7) | 22 (23.9) | 92 (100) |

As for vaccination status, nearly all children in the series (99%) were corrected vaccinated against H. influenzae in adherence with the current immunisation schedule of the autonomous community of Aragon, and 90% were vaccinated against pneumococcus (a vaccine not included in the routine immunization schedule until 2016).
The main symptom at diagnosis was fever, with a mean temperature of 38.7 ± 0.8 °C. In 70.6% of patients (95 CI: 60.6 to 78.9), the fever lasted 3 or more days, and the mean duration was 3.7 ± 1.9 days. Fever higher than 38 °C was more frequent in cases of probable pneumococcal pneumonia (93.3%) compared to atypical pneumonia (82.1%) and viral pneumonia (85%), although the differences were not statistically significant. Differences between atypical and viral cases were also not significant.
Frequent coughing was reported by 68.4% patients (95 CI: 58.4 a 77.0%) and cough was absent in 3.3%. The distribution by age of cough showed that children aged 25-60 months were less likely to report this symptom (4.6%), while severe cough was more prevalent in the group aged more than 60 months (77.2%).
Signs of respiratory distress were mostly absent (75%; 95 CI: 65.2 to 82.7). The most common ones were intercostal and subcostal retractions, found significantly more frequently in cases of viral vs atypical pneumonia (30% vs. 0%; OR: 3.0; 95 CI: 1.9 to 4.6; p = 0.0032) and in a low proportion of cases of probable pneumococcal pneumonia (16.1%).
The respiratory rate was recorded in 80 of the 92 patients. Tachypnoea was present in 47.5% of cases (95 CI: 36.9 to 58.3), and was significantly more frequent in cases of atypical pneumonia (69.2%) compared to probable pneumococcal bacterial pneumonia (35.7%) (OR: 4.0; 95 CI: 1.3 to 12.6; p = 0.0285), although the prevalence was similar in patients with viral pneumonia (35.2%) (p = 0.0605).
The oxygen saturation was measured in 84 out of the 92 patients, with a normal saturation of 95% or greater in 65.4% (95 CI: 54.8 to 74.7). Saturations at or below 92% were significantly more frequent in cases of viral pneumonia (26.3%) compared to probable pneumococcal pneumonia (3.2%) (OR: 10.7; 95 CI: 1.1 to 100.5; p = 0.0465) and also more frequent, although the difference was not statistically significant, than in cases of atypical pneumonia (12.5%).
Other signs and symptoms were less frequent, but it is important to take them into account in the diagnosis of CAP, such as gastrointestinal symptoms (18.4%) and pain in the ribs (5.4%), the latter of which was more frequent in cases of atypical pneumonia (10.7%) compared to cases of probable pneumococcal pneumonia (3.2%).
The most frequent radiographic pattern was the alveolar pattern (53.8%), followed by the mixed pattern (26.3%) and the interstitial pattern (19.7%). Atelectasis was found in 16.4% of cases. The most frequent location was the right hemithorax (45%). We did not find a statistically significant difference in the detection of the alveolar pattern between cases of atypical pneumonia (55.5%), probable pneumococcal pneumonia (54.8%) or viral pneumonia (50%). On the other hand, the interstitial pattern was more frequent in patients with atypical pneumonia (25.9%) and the mixed pattern in patients with viral pneumonia (30%). Figure 1 presents the distribution of radiographic patterns by type of pneumonia, evincing no differences. The mean age of patients with an alveolar pattern (50.6 ± 43.9 months) was not significantly greater compared to patients with an interstitial pattern (49.0 ± 38.6) (DM: 1.5 months; 95 CI: -20.9 to 24.0).
| Figure 1. Distribution of the most frequent radiographic patterns in each type of pneumonia (N = 91) |
|---|
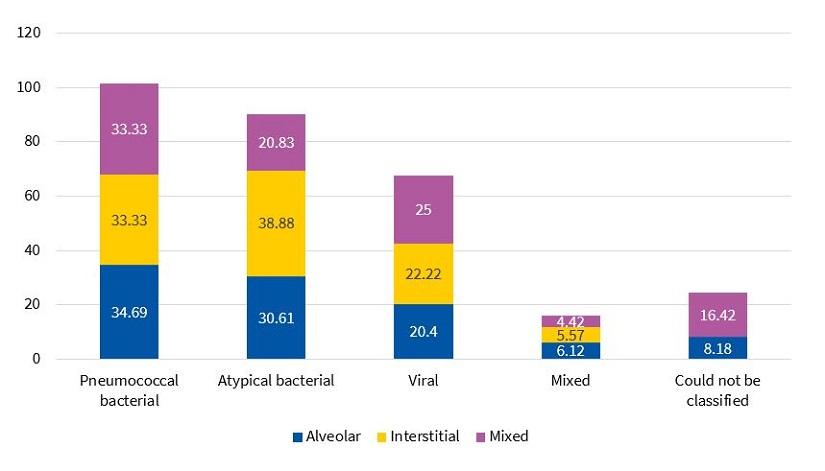 |

The mixed pattern was the most frequent pattern in cases of pneumonia caused by RSV (50%), while the alveolar pattern predominated in cases of pneumonia caused by influenza A virus (71.4%). Pleural effusion was found more frequently in patients with pneumococcal pneumonia (7.6%) and pneumonia by M. pneumoniae (7.4%) compared to viral pneumonia (0%).
Table 2 presents and compares laboratory test results. We found CPR levels greater than 6 mg/dl in 20% of patients (95 CI: 12.8 to 29.7), of who 41.1% had probable pneumococcal pneumonia and 41.1% viral pneumonia caused by RSV or influenza A virus. None of the patients with M. pneumoniae infection had CPR levels greater than 6 mg/dl. The elevation of CPR was greater in viral pneumonia (7.6 ± 9.5) compared to probable pneumococcal pneumonia (4.9 ± 10.1) (DM: -2.7; 95 CI: -8.3 to 3.1; p = 0.0490) and typical pneumonia (1.7 ± 1.7) (DM: -5.8; 95 CI: -10.4 to -1.1; p = 0.0111). We did not find differences between cases of probable typical and atypical bacterial pneumonia.
| Table 2. Distribution of white blood cell counts, neutrophil counts and serum CPR levels by type of pneumonia | |||
|---|---|---|---|
| Suspected pneumococcal pneumonia | Atypical bacterial pneumonia | Viral pneumonia | |
| White blood cell count (×1000) | n = 29 | n = 28 | n = 18 |
| Mean | 11 417 | 10 817 | 11 977 |
| Standard deviation | 5776 | 5015 | 5790 |
| Median | 10 300 | 9500 | 10 700 |
| Interquartile range | 4900-26 700 | 7250-17 000 | 78 200-22 600 |
| Neutrophil count (×1000) | n = 29 | n = 28 | n = 18 |
| Mean | 5387 | 5260 | 6644 |
| Standard deviation | 5229 | 3992 | 4756 |
| Median | 3300 | 4600 | 4600 |
| Interquartile range | 3700-25 400 | 3975-15 400 | 5075-17 100 |
| CPR (mg/dl) | n = 29 | n = 24 | n = 19 |
| Mean | 4.9 | 1.7 | 7.6 |
| Standard deviation | 10.1 | 1.7 | 9.5 |
| Median | 1.4 | 0.8 | 3.1 |
| Interquartile range | 4.1-46.5 | 2.1-5.5 | 9.0-31.4 |

The most frequent reasons for admission were signs of respiratory distress (45%), hypoxemia (35%), malaise (35%), multifocal involvement (30%) and failure of oral antibiotherapy (25%).
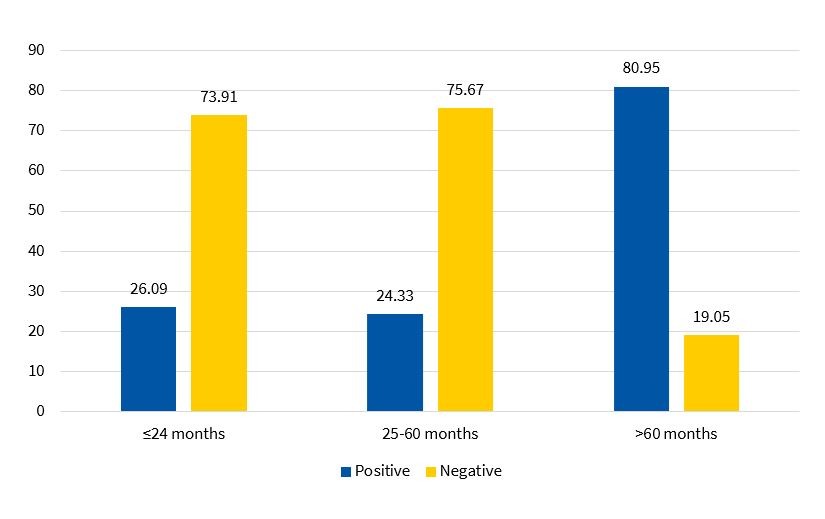
Serology tests found high titres of anti-M. pneumoniae IgM in 39.5% of cases. As can be seen in Figure 2, M. pneumoniae serology tests were mainly positive in children aged more than 5 years (80.9%), with a proportion of positive tests of 26.0% in children aged ≤2 years and of 24.3% in children aged 2-5 years.
Nasopharyngeal aspirate samples were obtained in 96 cases, with positive results in 30.2%. Table 3 presents the frequency distribution of the detected viruses, overall and by age group.
| Table 3. Distribution of viruses detected in nasopharyngeal aspirate samples by age group (N = 86) | ||||
|---|---|---|---|---|
| Virus | ≤24 months n (%) |
25-60 months n (%) |
>60 months n (%) |
Total n (%) |
| RSV | 5 (41.6) | 4 (40.0) | 1 (25.0) | 10 (38.4) |
| Influenza A | 2 (16.6) | 3 (30.0) | 2 (50.0) | 7 (26.9) |
| Influenza B | 0 (0) | 1 (10.0) | 0 (0) | 1 (3.8) |
| MPV | 2 (16.6) | 1 (10.0) | 1 (50.0) | 4 (15.3) |
| PIV 3 | 2 (16.6) | 0 (0) | 0 (0) | 2 (7.6) |
| Adenovirus | 1 (8.3) | 1 (10.0) | 0 (0) | 2 (7.6) |

Most patients started treatment with oral antibiotherapy, most frequently amoxicillin as monotherapy (66.3%; 95 CI: 56.1 to 75.1) followed by azithromycin (7.6%; 95 CI: 3.73 to 14.8), with a median duration of treatment of 7.3 ± 1.2 days for beta-lactam antibiotics and 3.5 ± 1.2 days for macrolides. Amoxicillin-clavulanic acid was only used for first-line treatment in 1% of patients. Intravenous antibiotherapy was given to 10.8% of patients (95 CI: 6.0 to 18.8), the drugs used most frequently for this purpose were ampicillin and cefotaxime, and the median duration of treatment was 3.5 ± 1.8 days.
The proportion of patients admitted to hospital was higher in cases of viral pneumonia (50%) compared to probable pneumococcal pneumonia (12.9%) (OR: 6.7; 95 CI: 1 to 26.50; p = 0.0100) and atypical pneumonia (7.1%) (OR: 13; 95 CI: 2.4 to 70.0; p = 0.0023).
Lastly, we conducted a multivariate analysis comparing the three main types of pneumonia. Table 4 presents the variables found to be significantly associated with aetiology. The mean age was higher in atypical pneumonia compared to probable pneumococcal pneumonia. Also, the proportions of patients with hypoxaemia and that required hospital admission were higher in the group with viral pneumonia compared to the group with probable pneumococcal pneumonia. The elevation of CPR was only significantly greater in viral pneumonia compared to atypical pneumonia.
| Table 4. Summary of the main results of the multivariate analysis comparing the 3 types of pneumonia | ||
|---|---|---|
| OR (95 CI) | p-value | |
| Suspected pneumococcal/typical bacterial | ||
| Age | 0.9 (0.8 a 0.9) | 0.013 |
| Age ≤2 years | 20.6 (1.8 a 228.1) | 0.014 |
| Suspected pneumococcal/viral | ||
| Age ≤2 years | 0.1 (0.01 a 0.9) | 0.0412 |
| Hypoxemia | 0.05 (0.01 a 0.7) | 0.0312 |
| Admission | 0.1 (0.03 a 0.6) | 0.0111 |
| Viral/atypical bacterial | ||
| Admission | 9.3 (1.5 a 56.9) | 0.0166 |
| CRP (mg/dl) | 1.3 (1.0 a 1.8) | 0.0365 |

DISCUSSION
Our study included a total of 92 patients, most of who were diagnosed at the primary care level and had a favourable outcome with empiric antibiotherapy. Paediatric cases of pneumonia diagnosed at the primary care level are usually milder, with lesser involvement of the lung parenchyma and a shorter course of disease. However, most studies in the literature focus on hospitalised patients with more complex disease and poorer outcomes.9-12
One fourth of our patients had a previous history of recurrent bronchitis and bronchial hyperresponsiveness, and nearly 10% a previous history of pneumonia, an already known risk factor that must be taken into consideration and identified in other case series.1,10,13,14
When it came to the aetiology of disease in our study, the most frequent type of pneumonia was probable pneumococcal pneumonia (33%), followed closely by atypical pneumonia (31%) and viral pneumonia (21%).
The proportions of atypical and viral pneumonia cases found in our study were substantial and consistent with the most recent data,2,15 suggesting that these aetiologies may be underdiagnosed in the paediatric population, as their actual incidence remains unknown.16 In our series, we found a high percentage of atypical and viral cases in children under 5 years, with a mean age below the age typically described in the literature. This high percentage may be due to earlier enrolment in school, but another possible contributor is that the serology tests used for diagnosis in our study measured IgM titres, which do not increase in episodes of reinfection, when there is a rapid IgG and IgA response instead, and patients were not tested for the presence of these other antibodies, which may have resulted in missed cases of reinfection in school-age children and adolescents.
Age was one of the main variables under study and, in agreement with the previous literature, probable typical pneumonia was more frequent in the 25-60 months age range, atypical pneumonia in children aged more than 60 months and viral pneumonia in children aged ≤24 months. Until recently, age was considered the strongest predictor of the type of pneumonia in the main clinical practice guidelines.5,6,9 As described in cases of atypical pneumonia in most series,9,14,17 patients with this type of pneumonia were older (74 ± 42 months), compared to the mean age of patients with viral pneumonia (36 ± 44 months) or probable pneumococcal pneumonia (32 ± 21 months).
When it came to the clinical manifestations, the most frequent symptoms were high fever, cough, tachypnoea and respiratory distress. Hypoxaemia was not common at the time of diagnosis. Fever was the most reported symptom, most frequently with a temperature of ≥39 °C and with a mean duration of 3.7 ± 1.9 days, consistent with the description given in different guidelines and by the WHO,6,8 with a high temperature ≥38.5 °C of more than 72 hours’ duration considered to support the diagnosis of community-acquired pneumonia.18 In our study, fever was a frequent finding in all types of pneumonia, but particularly frequent in cases of viral pneumonia caused by RSV, so its role as a predictor of a bacterial aetiology may be debatable.19
Severe cough was mainly present in children aged more than 5 years, and is considered a nonspecific symptom in the medical literature, albeit more frequent in older children and in patients of any age with a longer duration of disease,20 especially in case of viral pneumonia. It is important to take into account that cough is not usually present at onset of pneumonia, since alveoli have few cough receptors and therefore cough tends to develop when damage starts to occur and the detritus irritates cough receptors in the airways.
Half the patients presented with tachypnoea at diagnosis, the sign currently considered most specific for pneumonia in developing countries by different national and international guidelines.5,6,8 The proportion of patients with tachypnoea was greatest in the group with atypical pneumonia, especially compared to patients with probable pneumococcal pneumonia. Few studies have analysed this aspect,21 an interesting finding given that atypical pneumonia tends to have a more silent course, so that diagnosis may be delayed.
Excluding tachypnoea, only one fourth of patients showed signs of respiratory distress, as expected in a sample of cases of pneumonia managed at the primary care level, presumably in the early stages of disease. In our study, signs of respiratory distress were more severe in patients with viral pneumonia, especially compared to atypical pneumonia.
The oxygen saturation is an important marker of severity in children with any form of acute respiratory infection.4,22,23 We found a higher proportion of saturations of less than 92% in patients with viral pneumonia compared to pneumonia of other aetiologies, and specifically compared to probable typical pneumonia, an association that may be explained by the substantial bronchial involvement observed in cases of infection by viruses like RSV and by the lower age of the patients.
The most frequent radiographic pattern was the alveolar pattern, observed in half the patients, accompanied by atelectasis in 16%. Specifically, in the group of patients with an alveolar pattern, there was a similar proportion of atypical pneumonia and probable pneumococcal pneumonia cases, contrary to the classic patterns described in the past1,16 and in line with more recent studies that have not found any pattern as being chiefly associated with a specific aetiology.24,25 Variations in the radiographic pattern are also dependent on the age of the patient, with a predominance of the alveolar pattern in patients aged more than 5 years, the interstitial pattern in patients aged 2 to 5 years and a mixed pattern in children under 2 years.
The analysis of laboratory parameters revealed that CPR levels were significantly higher in cases of viral pneumonia compared to all other aetiologies. The highest elevations corresponded to cases caused by VRS and influenza A virus. However, we cannot attribute a viral aetiology based solely on this degree of CPR elevation, as some patients could have had bacterial coinfections, a possibility that we were not able to investigate due to the limitations of our study.
There are studies with similar limitations in resources that have used blood culture for diagnosis of pneumococcus. Since patients with pneumococcal pneumonia do not usually develop bacteraemia, the proportion of positive blood cultures is of less than 10%, so the diagnostic yield of blood culture in children is very low.26
Most patients in our case series received oral amoxicillin, which was prescribed correctly as monotherapy in 80% of cases of probable typical pneumonia, but was also prescribed in half of atypical and viral cases, with favourable outcomes. Only one fourth of cases of atypical pneumonia were treated with macrolides based on the working diagnosis, and we found no differences in outcomes compared to cases treated with beta-lactam antibiotics, which was consistent with the findings of some systematic reviews.27,28
As has been the case in most previous studies, the frequency of hospital admission neared 20%.1,9,13 The proportion of admitted patients was higher in the viral pneumonia group compared to the rest, probably due to the age of the patients and to the greater frequency of signs of respiratory distress.
One of the main limitations of our study, which was the inability to verify the diagnosis in one of the groups of pneumonia cases, is shared by most studies in the literature, and different studies have reported prevalences of viral pneumonia as high as 40% and a prevalence of atypical pathogens as high as 20-30% in an age range that deviates from previous descriptions.4,8,10
Another limitation concerns the statistical power achieved with the obtained sample size (N = 92), especially after dividing this sample into three main subgroups, possibly leading to results that were not statistically significant that could have been if a larger number of cases had been included. We applied a classification of the types of pneumonia that could be applicable given the limitations and lack of availability of aetiological diagnostic tests in the primary care setting. In the case of pneumococcal pneumonia, one of the challenges is that testing of NPA samples or antigen testing of urine samples cannot discriminate between colonization and disease, and may even give false positive results in recently vaccinated children, in addition to the low yield of blood culture. Therefore, the assumption in our study of a probable pneumococcal aetiology in cases with compatible clinical and radiographic features and negative for M. pneumoniae and other bacteria and viruses requires that results be interpreted with caution.
Lastly, the data in our study reveal few differential features of the types of pneumonia found in the paediatric population among the factors contemplated to date to guide the aetiological diagnosis.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article.
ABBREVIATIONS
CAP: community-acquired pneumonia · CPR: C-reactive protein · DM: difference of means · NPA: nasopharyngeal aspirate · OR: odds ratio · PC: Primary Care · RSV: respiratory syncytial virus · WHO: World Health Organization · 95 CI: 95% confidence interval.
FUNDING
This research project was partially funded by the 2018 José María Mengual Mur scholarship of the Fundación para el Progreso de la Pediatría.
REFERENCES
- Giménez Sánchez F, Sánchez Marenco A, Battles Garrido JM, López Soler JA, Sánchez-Solís Querol M. Características clínico-epidemiológicas de la neumonía adquirida en la comunidad en niños menores de 6 años. An Pediatr (Barc). 2007;66:578-84.
- Clark JE, Hammal D, Spencer D, Hampton F. Children with pneumonia: how do they present and how are they managed? Arch Dis Child. 2007;92:394-8.
- Lynch T, Bialy L, Kellner JD, Osmond MH, Klassen TP, Durec T, et al. A systematic review on the diagnosis of pediatric bacterial pneumonia: when gold is bronze. PLoS One. 2010;5:e11989.
- Shah SN, Bachur RG, Simel DL, Neuman MI. Does This Child Have Pneumonia? The Rational Clinical Examination Systematic Review. JAMA. 2017;318:462-71.
- Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children. Thorax. 2011;66:1-23.
- Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:25-76.
- Gerber JS, Prasad PA, Fiks AG, Localio AR, Grundmeier RW, Bell LM, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309:2345-52.
- The Management of acute respiratory infections in children: practical guidelines for outpatient care. In: World Health Organi zation [online] [accessed 07/09/2021]. Available at www.who.int/iris/handle/10665/41803.
- Úbeda Sansano MI, Murcia García J, Asensi Monzó MT, Grupo de Vías Respiratorias. Neumonía adquirida en la comunidad. El pediatra de Atención Primaria y la Neumonía. In: Asociación Española de Pediatría de Atención Primaria [online] [accessed 07/09/2021]. Available at www.aepap.org/sites/default/files/documento/archivos-adjuntos/protocolo-neumonia-2017.pdf
- Rudan I, O’Brien KL, Nair H, Liu L, Theodoratou E, Qazi S, et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. 2013;3:010401.
- Nair H, Simoes EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JS, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381:1380-90.
- GBD 2015 LRI Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1133-61.
- Andrés Martín A, Moreno-Pérez D, Alfayate Miguélez S, Couceiro Gianzo JA, García García ML, Korta Murua J, et al. Etiología y diagnóstico de la neumonía adquirida en la comunidad y sus formas complicadas. An Pediatr (Barc). 2012;76:162.e1-162.e18.
- Garcés-Sánchez MD, Díez-Domingo J, Ballester Sanz A, Peidro Boronat C, García López M, Antón Crespo V, et al. Epidemiología de la neumonía adquirida en la comunidad en menores de 5 años en la Comunidad Valenciana. An Pediatr (Barc). 2005;63:125-30.
- Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835-45.
- Rohde GGU. The role of viruses in CAP. Monograph 63: Community-Acquired Pneumonia. Eur Resp Monog. 2014;63:74-87.
- Jiang W, Wu M, Zhou J, Wang Y, Hao C, Ji W, et al. Etiologic spectrum and occurrence of coinfections in children hospitalized with community-acquired pneumonia. BMC Infectious Diseases. 2017;17:787-95.
- Michelow IC, Olsen K, Lozano J, Rollins NK, Duffy LB, Ziegler T, et al. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113:701-7.
- Moreno L, Krishnan JA, Duran P, Ferrero F. Development and validation of a clinical prediction rule to distinguish bacterial from viral pneumonia in children. Pediatr Pulmonol. 2006;41:331-7.
- NSW Health. Guideline. Infants and Children: Acute Management of Community Acquired Pneumonia. In: Agency for Clinical Innovation [online] [accessed 07/09/2021]. Available at www1.health.nsw.gov.au/pds/ActivePDSDocuments/GL2018_007.pdf
- Søndergaard MJ, Friis MB, Hansen DS, Jørgensen IM. Clinical manifestations in infants and children with M. pneumoniae infection. PLoS ONE. 2018;13:e019528.
- Mahabee-Gittens EM, Grupp-Phelan J, Brody AS, Donnelly LF, Allen Bracey SE, Duma EM, et al. Identifying children with pneumonia in the emergency department. Clin Pediatr (Phila). 2005;44:427-35.
- Smyth A, Carty H, Hart CA. Clinical predictors of hypoxaemia in children with pneumonia. Ann Trop Paediatr. 1998;18:31-40.
- Nelson KA, Morrow C, Wingerter SL, Bachur RG, Neuman MI. Impact of chest radiography on antibiotic treatment for children with suspected pneumonia. Pediatr Emerg Care. 2016;32:514-19.
- Finnegan OC, Fowles SJ, White RJ. Radiographic appearances of M. pneumoniae. Thorax. 1981;36:469-72.
- Hickey RW, Bowman MJ, Smith GA. Utility of blood cultures in pediatric patients found to have pneumonia in the emergency department. Ann Emerg Med. 1996;27:721-5.
- Mulholland S, Gavranich JB, Gillies MB, Chang AB. Antibiotics for community acquired lower respiratory tract infections secondary to M. pneumoniae in children. Cochrane Database Syst Rev. 2015;1:CD004875.
- Biondi E, McCulloh R, Alverson B, Klein A, Dixon A, Ralston S. Treatment of M. pneumoniae: a systematic review. Pediatrics. 2014;133:1081-90.