Prevalence of allergic diseases in parasitized children. Negative report of causality
Elías I. Ibrahim Kassissea, José Surga Félixb, Joselit Torres Bermúdezc, Jorge Elías Kassissed
aServicio de Neumología Infantil. Hospital Dr. Santos Aníbal Dominicci. Carúpano. Estado Sucre. Venezuela. Hospital Clínico Herminda Martín. Chillán. Región del Ñuble. Chile .
bMIR. Posgrado de Puericultura y Pediatría. Sahuapa. Cumaná. Estado de Sucre. Venezuela.
cPediatra inmunólogo. Sociedad Venezolana de Alergia, Asma e Inmunología. Caracas. Venezuela.
dMédico rural. Hospital tipo I Dr. Alberto Musa Yibirin. El Pilar. Estado de Sucre. Venezuela. CESFAM Villa Prat. Sagrada Familia. Curicó. Región del Maule. Chile.
Correspondence: EII Kassisse. E-mail: ekassisse@gmail.com
Reference of this article: Kassisse EII, Surga Félix J, Torres Bermúdez J, Kassisse JE. Prevalence of allergic diseases in parasitized children. Negative report of causality. Rev Pediatr Aten Primaria. 2020;22:e111-e119.
Published in Internet: 24-07-2020 - Visits: 15276
Abstract
Introduction: study of the relationship between intestinal parasites and atopic diseases, such as asthma or rhinoconjunctivitis, has produced contradictory results. The objective of this study was to evaluate the relationship between intestinal parasitosis and the prevalence of atopic diseases.
Material and method: we conducted an observational, cross-sectional field study in children aged more than 2 years whose parents completed the questionnaires for evaluation of the presence of atopic diseases. We performed serial stool sample examinations and skin allergy tests in every child in the sample. The statistical analysis was carried out using the χ2 test with calculation of odd ratios and 95% confidence intervals. We defined statistical significance as p <0.05.
Results: we evaluated 185 children aged 6.82±2.69 years, 52% male, 94% in the socioeconomic status category III/IV. The prevalence of atopic disease of any kind was 41%, 43% had positive results of skin allergy testing and parasites of any type were detected in 47%. We found a significant association between the results of the ISAAC questionnaire and the results of the skin prick test (p = 0.000). We did not find a significant association between the results of the skin prick test and the detection of intestinal parasitic infection (p = 1.29), or between the results of the ISAAC questionnaire and parasitic infection (p = 0.447).
Conclusion: the findings of our study suggest that the presence of allergic conditions is not associated with the diagnosis of parasitic infection.
Keywords
● Asthma ● Atopic disease ● Helminthiasis ● Parasitic infection ● Skin allergy testsINTRODUCTION
The prevalence of allergic diseases such as asthma, rhinoconjunctivitis (RC) or atopic eczema (AE) is believed to be as high in the tropics as elsewhere in the world, and many risk factors are common to both temperate and tropical regions, but there are 2 risk factors that differentiate these geographical areas: one is the persistent exposure to house dust mites, and the other are helminth infections.1
The effect of living conditions and infections in the prevention and atopy and asthma is encompassed in the concept that has come to be known as the hygiene hypothesis, which is based on the inverse correlation found between childhood infections and the prevalence of these allergic diseases.2,3
The main environmental factors contemplated in the hygiene hypothesis are residence in a rural area, which is associated with agricultural activity and exposure to animals, and parasitic infections.4,5
Helminth infections frequently involve geohelminths such as Ascaris lumbricoides, Trichuris trichiura and Ancylostoma species (Necator americanus and Ancylostoma duodenalis). These pathogens are found worldwide, and it is estimated that approximately one fourth of the global population may be infected.6
Helminths have antigens that induce an inflammatory allergic response in the host manifesting with a high production of IgE and cytokines (predominantly IL-4, IL-5 and IL-13), which characterises a Th2 inflammatory immune response with an elevated eosinophil count.7
The interaction between helminth infection and atopy is mediated by multiple factors, which include the timing and duration of the initial infection, the severity of infection, host genetic factors and the type of parasite.8,9
The association between intestinal parasitic infection and allergic diseases has not been well established and continues to be controversial. On one hand, there is evidence suggesting that parasitic infections may have a protective effect against the development of allergic disease, while on the other a high parasite load has been shown to be a risk factor for allergy and to contribute to the high prevalence of asthma and other allergic diseases in childhood.10-12 This association with allergy has also been investigated for protozoans such as Giardia lamblia.13.
The aim of this study was to establish the association between intestinal parasitic infections and allergic diseases in a group of schoolchildren in an urban setting.
MATERIAL AND METHODS
We conducted an observational, cross-sectional field study in the early childhood, primary and secondary public education population of the Municipality of Sucre in the state of Sucre, Venezuela, in the academic year from October 1, 2016 to July 30, 2017.
We included all children aged more than 2 years whose parents completed the form designed to determine the prevalence of allergic disease and that underwent serial stool examinations.
We excluded children that had used any antihelminthic medication or any other antiparasitic drugs in the past 4 weeks, children that were not regular students in the local public school system and children that had used antihistaminic drugs in the 7 days preceding the skin allergy tests.
For each participant, we collected 3 serial stool samples in consecutive days in plastic containers with a lid, clean and properly labelled with the name and age of the participant, which were immediately transported to the laboratory in refrigerated boxes, due to the lack of instant fixative solutions, at a temperature ranging from 2°C to 6°C for subsequent analysis.
Parasitic detection in stool was performed by direct examination of a stool sample in normal saline (0.9%) and Lugol’s iodine (1%) solution. A positive result was defined as detection of any helminth or protozoan.
We estimated the prevalence of asthma based on the answers to items 1 and 2 of the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire, validated in Spanish, which ask about the presence of wheezing any time in the past and in the past 12 months.14
We also estimated the prevalence of rhinoconjunctivitis based on the phase 3 ISAAC questionnaire version for Latin America, and defined a positive history of allergic rhinitis as a “yes” answer to the items: “In the past 12 months, have you /has your child ever had a problem with sneezing, or a runny or blocked nose when you/he/she Yes DID NOT have a cold or the flu?” and “In the past 12 months, has this nose problem been accompanied by itchy-watery eyes?”.15
We also used the phase 3 ISAAC questionnaire in Spanish to establish the presence of eczema, considering “yes” answers as a measure of prevalence: “Has your child had this itchy rash at Yes any time in the past 12 months?” and “Has this itchy rash at any time affected any of the following places: the folds of the elbows, behind the knees, in front of the ankles, under the buttocks, or around the neck, ears or eyes?”.16
We assessed for the presence of allergy with the skin prick test (SPT) method, using the Inmunotek® system (Madrid, Spain), which delivers a standardised amount of allergen diluted in 50% glycerine and 0.5% phenol, using histamine for the positive control and 0.9% saline solution for the negative control; the tests used allergens from Dermatophagoides, Blomia tropicalis, fungi, cat, dog, mosquito, cockroach (Periplaneta americana), trigo, white fish, oily fish, orange, cacao, egg white, egg yolk, cow’s milk and shellfish. The interpretation adhered to international guidelines. Fifteen minutes after applying the antigen, the wheal was measured and the test considered positive if the wheal was the same size or larger than the respective control. We expressed the results with qualitative categories: + = <½ control; ++ = ½ control; +++ = control; ++++ ≥ control; and +++++ = 3 times the size of the control.17
We classified participants by socioeconomic status based on the scale developed by Graffar-Méndez.18
We calculated descriptive statistics (mean, range and standard deviation), made comparisons by means of the χ2 test and calculated odds ratios (ORs); statistical significance was defined as p <0.05.
The study protocol was evaluated and approved by the Committee on Postgraduate Education of the Hospital Antonio Patricio de Alcalá, which deemed that the study adhered to the ethical principles of the Declaration of Helsinki. We obtained the informed consent of all participants.
RESULTS
We distributed 284 questionnaires, and we received 185 (65%) back with all the necessary information completed, we excluded 7 due to children not completed the serial stool examinations and 48 due to children not showing up on the day that the skin prick tests were performed, so the final sample included 130 children.
The mean age of the sample was 6.82 ± 2.69 years, the overall prevalence of allergic diseases was 41%, and parasites (of any kind) were detected in 47% of the total sample. Table 1 summarises these data.
| Table 1. General characteristics of the study sample | |||||||
|---|---|---|---|---|---|---|---|
| Variable* | Parasitic infection | ||||||
| Infection (n = 61) |
No Infection (n = 69) |
Total (n = 130) |
OR | CI | p | ||
| Age (years) | 6.66 ± 2.704 (3-12) |
7.02 ± 2.692 (3-12) |
6.82 ± 2.698 (3-12) |
||||
| Sex | Male | 40 (30.8) | 28 (21.5) | 68 (52.3) | 2.789 | 1.366-5.696 | 0.005 |
| Female | 21 (16.2) | 41 (31.5) | 62 (47.7) | ||||
| Graffar scale | I | 0 (0) | 0 (0) | 0 (0) | |||
| II | 1 (0.8) | 6 (4.6) | 7 (5.4) | ||||
| III | 32 (24.6) | 44 (33.8) | 76 (58.5) | 0.627 | 0.311-1.266 | 0.215 | |
| IV | 28 (21.5) | 19 (14.6) | 47 (36.2) | 2.233 | 1.076-4.632 | 0.044 | |
| V | 0 (0) | 0 (0) | 0 (0) | ||||
| ISAAC | Positive | 20 (15.4) | 33 (25.4) | 53 (40.8) | 0.532 | 0.261-1.086 | 0.108 |
| Negative | 41 (31.5) | 36 (27.7) | 77 (59.2) | ||||
| Age group | Early childhood | 20 (15.4) | 21 (16.2) | 41 (31.5) | 0.083 | ||
| School-aged | 41 (31.5) | 48 (36.9) | 89 (68.5) | ||||
| Skin prick test | Positive | 22 (16.9) | 34 (26.2) | 56 (43.1) | 0,581 | 0.287-1.174 | 0.157 |
| Negative | 39 (30) | 35 (26.9) | 74 (56.9) | ||||
| Total | 61 (46.9) | 69 (53.1) | 130 (100) | ||||

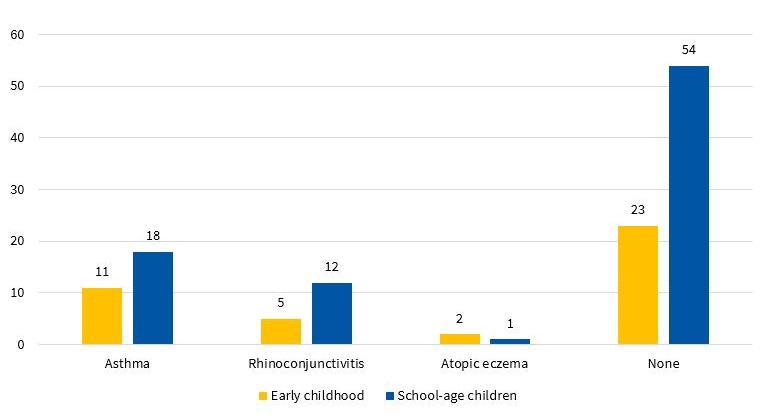
The prevalence of asthma was 22.3%, and the prevalence of RC 13.1%, and both of these percentages increased with age, while the prevalence of AE was 2.4%. We only found a combination of these diseases in 3% of the children (Figure 1).
| Figure 1. Results of the ISAAC questionnaire and their association with age group. Asthma prevalence of 22.3% |
|---|
 |

The skin prick tests were positive in 56 children, corresponding to a prevalence of 43.1%; the test was most frequently positive for a single allergen (30%).
The allergens identified most frequently were dust mites (Dermatophagoides, Blomia tropicalis), found in 33/56 children (58.9%), followed by mosquitos (17.8%) and cockroaches (12.5%), and few had a positive result for a foodstuff (1.8%).
At least one parasite was detected in 61 children, corresponding to a prevalence of 47%; 40 of these participants were male (40/61), and there was a predominance of protozoans (27%) and helminths (13.1%), while 9.2% of children were infected by more than one parasite.
Blastocystis hominis was the protozoan detected most frequently (10.8%), followed by Giardia lamblia, while the most frequent helminth was Ascaris lumbricoides (6.9%).
We did not find a statistically significant association between age and parasite infection (p = 0.083) or between age and they type of parasite detected (χ2 = 0.65<7.81(3; 0.05), p = 0.883), but we found an association between the Graffar category and parasitic infection, with children in the category corresponding to the lowest socioeconomic status level (IV) exhibiting a significantly greater prevalence of parasitic infection (χ2 = 6.72 > 5,99(2; 0.05)); children in this level were at least twice as likely to have a parasitic infection (OR = 2.23; 95% confidence interval [95 CI]: 1.07 a 4.63; p = 0.004) (Table 1), although the socioeconomic level was not associated with the type of parasite (χ2 =7.71 < 12.59(6; 0,05), p = 0.260).
In male participants, the prevalence of parasitic infection was greater (χ2 = 8.10 > 6.63(1; 0.01); p = 0.004) and helminths or protozoans were detected more frequently compared to female participants (χ2 = 8,58 > 7,81(3; 0,05); p = 0.035; OR = 2,78; 95 CI: 1,36 a 69; p = 0.005]) (Table 1).
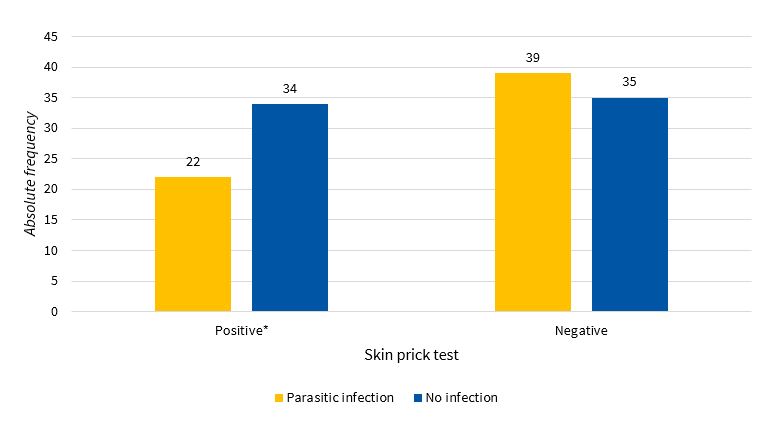
We did not find a statistically significant association (χ2 = 2.30 < 3.84(1; 0,005), p = 1.29) between a positive result of skin allergy testing and the presence of parasitic infection, but we ought to highlight that more than half of children with a positive skin prick test were not infected (Table 1, Figure 2). Although we did not find significant differences, we assessed the risk of having a positive skill allergy test result based on the type of parasite found most frequently, and found that the risk of a positive test was nearly twofold in children with infection by Giardia lamblia compared to children with infection by Ascaris lumbricoides (OR = 1.14; 95 CI: 0.36 a 3.62; p = 0.813 versus OR = 0.16; IC 95: 0.02 a 1.36; p = 0.058).
| Figure 2. Results of the skin prick test and their association with the detection of any type of parasite |
|---|
 |

The results of the ISAAC questionnaire were not significantly associated with the presence of parasitic infection (χ2 = 3.03 < 3.84(1; 0.05). p = 0.082) or the type of parasite (χ2 = 3.59 < 7.81(3; 0.05), p = 0.447) (Table 1).
However, we found a clearly significant association (χ2 = 42.88 > 10.82(1; 0.01). p = 0.000) when we compared the results of the ISAAC questionnaire with the results of the skin allergy tests, as children with a positive ISAAC questionnaire had an up to 14-fold risk of having a positive skin test, with this risk increasing with age (χ2 = 4.14 > 3.84(1; 0.05). p = 0.042; OR: 14.12; IC 95: 6.00 a 33.22. p = 0.000 y OR: 2.16; IC 95: 1.02 a 4.59. p = 0.042) (Table 2).
| Table 2. Association between skin prick tests, age, sex and ISAAC results | ||||||
|---|---|---|---|---|---|---|
| Prick test | OR | IC | p | |||
| Positive* | Negative* | |||||
| n (%) | n (%) | |||||
| Age | Early childhood | 23 (17.7) | 18 (13.8) | 2.168 | 1.022-4.599 | 0.042 |
| School-aged | 33 (25.4) | 56 (43.1) | ||||
| Sex | Male | 25 (19.2) | 43 (33.1) | 0.581 | 0.289-1.171 | 0.128 |
| Female | 31 (23.8) | 31 (23.8) | ||||
| ISAAC | Positive | 41 (31.5) | 12 (9.2) | 14.122 | 6.003-33.223 | 0.000 |
| Negative | 15 (11.5) | 62 (47.7) | ||||
| Total | 56 (43.1) | 74 (56.9) | ||||

We did not find a significant association of a positive skin prick test result with sex (χ2 = 2.31 < 3.84(1; 0.05). p = 0.128), the Graffar socioeconomic status level (χ2 = 1.60 < 5.99(1; 0.05). p = 0.447) (Table 2) or the presence of parasites (χ2 = 3.57 < 7.81(3; 0.05). p = 0.311) (Table 1).
DISCUSSION
Our observational study, designed to assess the association between atopy, allergic disease and intestinal parasite infection, did not find any such associations.
Different studies comparing various conditions to the presence of geohelminth infection continue to yield contradictory results. For example, the studies by Bragagnoli et al.10, Webb et al.12 and Endara et al.19 suggest that the parasite load or the mere presence of intestinal parasites are directly correlated with the presence of allergic manifestations, a risk factor that contributes to the high prevalence of asthma and related symptoms. On the other hand, a study by Leonardi-Bee et al.20 suggested that parasitic infections generally do not protect against asthma, and, more recently, Cooper et al.21 found evidence that the decreased risk of wheezing and asthma in children with parasitic infection was due to a non-allergic mechanism.
Our findings suggest that the prevalence of asthma in our population is high and its aetiology is frequently allergic, despite the high proportion of children with parasitic infection, which suggests a negative association and the lack of a protective effect on atopy.
A study in 1004 children found that 260 had infection by A. lumbricoides and 233 had asthma. The parasite load was directly associated with episodes of recurrent wheezing. The prevalence of wheezing was much higher in children with higher parasite loads (p = 0.003, OR = 0.41, 95 CI: 0.22 to 0.75), which suggests that parasite loads this high constitute a risk factor that contributes to the high prevalence of asthma and related symptoms.10
Similarly, a cross-sectional study showed that individuals with parasitic infection were more likely to have atopic conditions.12
These studies contrast with another study that compared two populations, one rural and one urban, in Ecuador. This study found a strong association between sensitization to house dust and wheezing in children residing in urban settings and that this difference was attenuated and explained by the significantly higher prevalence of geohelminths in the rural population.19
Helminths have not been the only parasites whose association with allergy has been studied, as protozoans have been investigated as well. A study in children residing in an urban area detected the presence of Giardia lamblia in 45%, but this was not associated with allergic symptoms or the results of skin allergy testing.22
Socioeconomic status is one of the variables that has been found to have an effect on the prevalence of allergic diseases.23,24
A study in a sample of 481 children of low socioeconomic status that assessed the risk factors associated with allergy test results found factors that could perform as protectors and that supported the hygiene hypothesis.25
Our analysis of socioeconomic level allowed us to determine that the socioeconomic status of the population of schoolchildren under study was low (Graffar III/IV). While we were unable to establish an association with atopy, we ought to highlight that the probability of parasitic infection was doubled in children of low socioeconomic status.
In a study in Cuban children, Wördemann et al.26 found that current infection by A. lumbricoides had a protective effect against atopic dermatitis, while past infection by E. vermicularis and Ancylostoma species rather acted like risk factors for development of rhinoconjunctivitis and atopic dermatitis, which suggests that the impact of infection varies depending on the type of parasite and timing of infection even within a single individual.
In our study, a positive ISAAC questionnaire was strongly associated with the results of skin allergy tests.
This finding was consistent with those of a study in Germany, which found a very high negative predictive value (0.94) and a low positive predictive value (0.09) of the skin prick test, which means that it is very unlikely for a negative test result to be associated with the presence of asthma.27
The association found in our study between male sex and an increased risk of parasitic infection has not been found in other studies.28,29
An interesting aspect to consider that could be explored in future research is the association of sex hormones and their impact on immune responses and on the development of allergy in individuals with parasitic infections, which may entail an increased resistance of girls to parasitic infection.
We ought to mention some of the limitations of our study. The chief limitation may be the sample, although we consider that the sample was sufficiently representative to draw conclusions. Other important limitations are that we did not use concentration procedures in our analysis of the magnitude of infection and that we did not know the previous infection status of participants.
In conclusion, our study did not support the protective effect of parasitic infections in relation to diagnosis of allergic diseases or an association of these infections with the results of skin allergy tests.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article.
ABBREVIATIONS
AE: atopic eczema · CI: confidence interval · ISAAC: International Study of Asthma and Allergies in Childhood · OR: odds ratio · RC: rhinoconjunctivitis · SPT: skin prick test.
REFERENCES
- Zakzuk J, Lee BW, Acevedo N, Soh JY, Sánchez-Borges M, et al. Particularities of allergy in the Tropics. World Allergy Organ J. 2016;9:20.
- Briggs N, Weatherhead J, Sastry KJ, Hotez PJ. The hygiene hypothesis and its inconvenient truths about helminth infections. PLoS Negl Trop Dis. 2016;10:e0004944.
- Daley D. The evolution of the hygiene hypothesis: the role of early-life exposures to viruses and microbes and their relationship to asthma and allergic diseases. Curr Opin Allergy Clin Immunol. 2014;14:390-6.
- Von Mutius E. The microbial environment and its influence on asthma prevention in early life. J Allergy Clin Immunol. 2016;137:680-9.
- Maizels RM, McSorley HJ. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol 2016;138:666-75.
- Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521-32.
- Yasuda K, Nakanishi K. Host responses to intestinal nematodes. Int Immunol. 2018;30:93-102.
- Cooper PJ, Barreto M, Rodrigues LC. Human allergy and geohelminth infections: a review of the literature and a proposed conceptual model to guide the investigation of possible causal associations. Br Med Bull. 2006;79-80:203-18.
- Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M. Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis. 2014;pii:S1473-3099.
- Bragagnoli G, Silva MT. Ascaris lumbricoides infection and parasite load are associated with asthma in children. J Infect Dev Ctries. 2014;8:891-7.
- Stein M, Greenberg Z, Boaz M, Handzel ZT, Meshesha MK, Bentwich Z. The role of helminth infection and environment in the development of allergy: a prospective study of newly-arrived ethiopian immigrants in Israel. PLoS Negl Trop Dis. 2016;10:e0004208.
- Webb EL, Nampijja M, Kaweesa J, Kizindo R, Namutebi M, Nakazibwe E, et al. Helminths are positively associated with atopy and wheeze in Ugandan fishing communities: results from a cross-sectional survey. Allergy. 2016;71:1156-69.
- Souza VM, Sales IR, Peixoto DM, Costa VM, Rizzo JA, Silva AR, et al. Giardia lamblia and respiratory allergies: a study of children from an urban area with a high incidence of protozoan infections. J Pediatr (Rio J). 2012;88:233-8.
- Mallol J, Solé D, Baeza-Bacab M, Aguirre-Camposano V, Soto-Quiros M, Baena-Cagnani C; et al. Regional variation in asthma symptom prevalence in Latin American children. J Asthma. 2010;47:644-50.
- Solé D, Mallol J, Camelo-NunesI C, Wandalsen GF; Latin American ISAAC Study Group. Prevalence of rhinitis-related symptoms in Latin American children - results of the International Study of Asthma and Allergies in Childhood (ISAAC) phase three. Pediatr Allergy Immunol. 2010;21:e127-36.
- Solé D, Mallol J, Wandalsen GF, Aguirre V; Latin American ISAAC Phase 3 Study Group. Prevalence of symptoms of eczema in Latin America: results of the International Study of Asthma and Allergies in Childhood (ISAAC) Phase 3. J Investig Allergol Clin Immunol. 2010;20:311-23.
- Heinzerling L, Mari A, Bergmann KC, Bresciani M, Burbach G, Darsow U, et al. The skin prick test – European standards. Clin Transl Allergy. 2013;3:3.
- Méndez C. Estudio nacional de crecimiento y desarrollo humano de la República de Venezuela. Caracas: Proyecto Venezuela; 1996.
- Endara P, Vaca M, Platts-Mills TA, Workman L, Chico ME, Barreto ML, et al. Effect of urban vs. rural residence on the association between atopy and wheeze in Latin America: findings from a case-control analysis. Clin Exp Allergy. 2015;45:438-47.
- Leonardi-Bee J, Pritchard D, Britton J. Asthma and current intestinal parasite infection: systematic review and meta-analysis. Am J Respir Crit Care Med. 2006;174:514-23.
- Cooper PJ, Chico ME, Vaca MG, Sandoval CA, Loor S, Amorim LD, et al. Effect of early-life geohelminth infections on the development of wheezing at 5 years of age. Am J Respir Crit Care Med. 2018;197:364-72.
- Jansson SA, Protudjer JL, Arnlind Heibert M, Bengtsson U, Kallström-Bengtsson I, Marklund B, et al. Socioeconomic evaluation of well-characterized allergy to staple foods in adults. Allergy 2014;69:1241-7.
- Thakur N, Martín M, Castellanos E, Oh SS, Roth LA, Eng C, et al. Socioeconomic status and asthma control in African American youth in SAGE II. J Asthma. 2014;51:720-8.
- Hamid F, Wahyuni S, van Leeuwen A, van Ree R, Yazdanbakhsh M, Sartono E. Allergic disorders and socio-economic status: a study of schoolchildren in an urban area of Makassar, Indonesia. Clin Exp Allergy. 2015;45:1226-36.
- Alcantara-Neves NM, Veiga RV, Ponte JC, da Cunha SS, Simões SM, Cruz ÁA, et al. Dissociation between skin test reactivity and anti- aeroallergen IgE: Determinants among urban Brazilian children. PLoS One. 2017;12:e017408.
- Wördemann M, Díaz RJ, Heredia LM, Collado Madurga AM, Ruiz Espinosa A, Prado RC, et al. Association of atopy, asthma, allergic rhinoconjunctivitis, atopic dermatitis and intestinal helminth infections in Cuban children. Trop Med Int Health. 2008;13:180-6.
- Gallmeier K, Becker E, Kirsten A, Wölke G, Manuwald O, Meyer H, et al. Prediction of new-onset asthma and nasal allergy by skin prick test and RAST in a cohort of adults. Eur Respir J. 2014;43:92-102.
- Nxasana N, Baba K, Bhat V, Vasaikar S. Prevalence of intestinal parasites in primary school children of Mthatha, eastern cape province, South Africa. Ann Med Health Sci Res. 2013;3:511-6.
- Chin YT, Lim YA, Chong CW, Teh CS, Yap IK, Lee SC, et al. Prevalence and risk factors of intestinal parasitism among two indigenous sub-ethnic groups in Peninsular Malaysia. Infect Dis Poverty. 2016;5:77.






