Possible relationship between alopecia areata and enterovirus infections
Santiago Conde Barreiroa, Beatriz González Pelegrínb, Eduardo Clemente Roldánc, Maria Pilar Rodrigo Vald, Alfredo Yuste Arae
aPediatra. CS de Barbastro. Barbastro. Huesca. España.
bEnfermera. Hospital de Barbastro. Barbastro. Huesca. España.
cMedicina Preventiva. Hospital de Barbastro. Barbastro. Huesca. España.
dEspecialista en Medicina Preventiva. Dirección General de Salud Pública del Gobierno de Aragón. Zaragoza. Zaragoza. España.
eTécnico Informático. Hospital de Barbastro. Barbastro. Huesca. España.
Correspondence: S Conde. E-mail: santycon@terra.com
Reference of this article: Conde Barreiro S, González Pelegrín B, Clemente Roldán E, Rodrigo Val M, Yuste Ara A. Possible relationship between alopecia areata and enterovirus infections. Rev Pediatr Aten Primaria. 2014;16:e87-e93.
Published in Internet: 07-10-2014 - Visits: 48569
Abstract
Introduction: alopecia areata is an autoimmune disease of unknown etiology that is associated to other autoimmune diseases. Hand-foot-mouth disease is a common viral infection in children caused by several enterovirus serotypes, and it is sometimes associated to onychomadesis. This study intends to investigate an increase in cases of alopecia areata after an outbreak of hand-foot-mouth disease in our environment.
Methods: identification of patients under 14 years old diagnosed with hand-foot-mouth disease and/or alopecia areata in a Primary Care Service Area between 1/1/2011 and 31/12/2012. Review of medical records, collecting date of birth and diagnosis, age at diagnosis and clinical characteristics.
Results: forty-nine patients diagnosed with hand-foot-mouth disease and 7 diagnosed with alopecia areata were found. An outbreak of hand-foot-mouth disease was confirmed with 42 cases within a period of 8 weeks. A subsequent increase in cases of alopecia areata (4 cases within 4 weeks, compared with 3 cases over the 11 months before and none during the previous year) was observed.
Conclusions: following an outbreak of hand-foot-mouth disease an increased number of cases of alopecia areata was observed. This fact, coupled with the association of alopecia areata with other autoimmune diseases, and the relationship found between enterovirus infections and autoimmune diseases such as type 1 diabetes mellitus poses a possible causal relationship between enterovirus infections and alopecia areata, and a possible component of autoimmunity in onychomadesis associated to hand-foot-mouth disease.
Keywords
● Alopecia areata ● Autoimmunity ● Enterovirus ● Hand, Foot and mouth disease ● Onychomadesis ● PediatricsNote:
How to quote this article:
Conde Barreiro S, González Pelegrín B, Clemente Roldán E, Rodrigo Val MP, Yuste Ara A. Posible relación entrealopecia areata e infecciones por enterovirus. Rev Pediatr Aten Primaria. 2014;16:212.e87-e93.
INTRODUCTION
Alopecia areata is an autoimmune disorder of unknown and probably multifactorial aetiology. Its pathogenesis involves genetic, immunologic, infectious, circulatory, neurologic, and psychogenic factors. It is frequently associated with other autoimmune diseases, such as vitiligo, type 1 diabetes mellitus, Hashimoto’s thyroiditis, Addison’s disease or pernicious anaemia, and also to diseases associated with hypersensitivity, such as asthma and atopic dermatitis.1-3
Up to 20% to 30% of these patients have a family history of the disease, a percentage that is even higher in early-onset cases. It has been hypothesised that these heredity factors are associated with HLA complex genes, and haplotypes that predispose individuals to the disease have been described in the literature.4
When it comes to infectious factors, the literature has described potential associations with infection by Epstein-Barr virus, cytomegalovirus, the varicella-zoster herpesvirus, swine flu virus and Helicobacter pylori, although these relationships have yet to be confirmed.5-7 An association between alopecia areata and enterovirus infections has not yet been described in the literature, although it is suspected that some enteroviruses may be involved in the aetiopathogenesis of autoimmune diseases such as type 1 diabetes mellitus.8-9
Hand, foot, and mouth disease (HFMD) is a frequent infectious illness in the paediatric age group, characterised by the development of fever, mouth sores, and vesiculopapular exanthema predominantly found in distal regions of the limbs (hands and feet). The illness is caused by various enteroviruses, usually coxsackievirus A16 and EV71, but also coxsackievirus A5, A7, A9, A10, B1, B2, B3 and B510 andechovirus E3, E4 and E9.11 Cases may be sporadic or occur in small outbreaks in child care centres and schools, usually in late spring and autumn. In recent years the literature has described outbreaks of disease associated with coxsackievirus A6, often with atypical clinical manifestations such as a different distribution of the lesions, greater size of lesions in some patients (including presentations with extensive skin involvement such as eczema coxsackium) and a higher incidence in adults,12-14 as well as outbreaks caused by EV71 in South East Asia with more severe forms of the disease and some of the infections resulting in death.15
Furthermore, it has been established in recent years that there is an association between HFMD and onychomadesis. This finding, which stemmed from the observation that cases of onychomadesis developed shortly after outbreaks of HFMD in specific geographical areas, was first reported in Chicago10 and later corroborated in different regions across the world,16,12,14 including some in Spain.11,17-19 Onychomadesis usually occurs in children younger than 3 years between 4 and 8 weeks after HFMD,19 and develops more frequently when the latter is caused by coxsackievirus A6.12,14 Cases in older children and even adults exposed to coxsackievirus A6 have also been reported.20 At present the mechanisms involved in the aetiopathogenesis of onychomadesis are unknown, although it has been suggested that it may result from direct damage to the nail matrix by the virus,14 nail matrix arrest due to the inflammation of papulovesicular lesions close to the nail matrix, the systemic impact of the disease, or damage secondary to fungal infections fostered by excessive hygiene in affected areas.21
In 2012 an outbreak of HFMD with atypical characteristics in our health area was followed by an increased number of alopecia areata diagnoses in the paediatric age group.
The aim of our study was to verify whether there had been an increased number of diagnosed alopecia areata cases following the HFMD outbreak in our area, and to describe the characteristics of the observed cases to determine whether there is a potential association between the two conditions.
MATERIALS AND METHODS
We performed a descriptive study. We searched the primary care electronic records database (OMI-AP) for patients younger than 14 years diagnosed with HFMD and/or alopecia in our health area between January 1, 2011 and December 31, 2012. We reviewed the medical records of the identified patients and their outcomes up to June 30, 2013 (six months after the end of the study period). We selected only those alopecia cases with clinical features suggestive of alopecia areata, excluding other types of alopecia (trichotillomania, hair loss without areas of alopecia, etc). We collected data for the date of birth, date of diagnosis, and age at diagnosis. We reviewed the characteristics of the cases documented in the medical history and the microbiology tests performed. In the statistical analysis we analysed the frequency distribution of cases by years and weeks, the mean age at diagnosis, and the range of age at diagnosis for the HFMD cases documented to have occurred during the outbreak and for the cases of alopecia areata documented in the weeks following the outbreak. We made the statistical analysis using Excel® 97 and SPSS® 15.0.
RESULTS
We found a total of 49 paediatric patients diagnosed with HFMD and 7 patients diagnosed with alopecia areata during the study period.
We identified 3 patients that had been diagnosed with HFMD and none diagnosed with alopecia areata in 2011, while we found 46 patients diagnosed with HFMD and 7 diagnosed with alopecia areata in 2012.
We confirmed that there had been an outbreak of HFMD, with a total of 42 diagnosed cases in an 8-week period (October 15–December 5, 2012), with the highest number of cases diagnosed in the fourth week of the outbreak (n = 10). The mean age of the patients was 21 months (standard deviation, 12.21 months; range, 8-59 months).
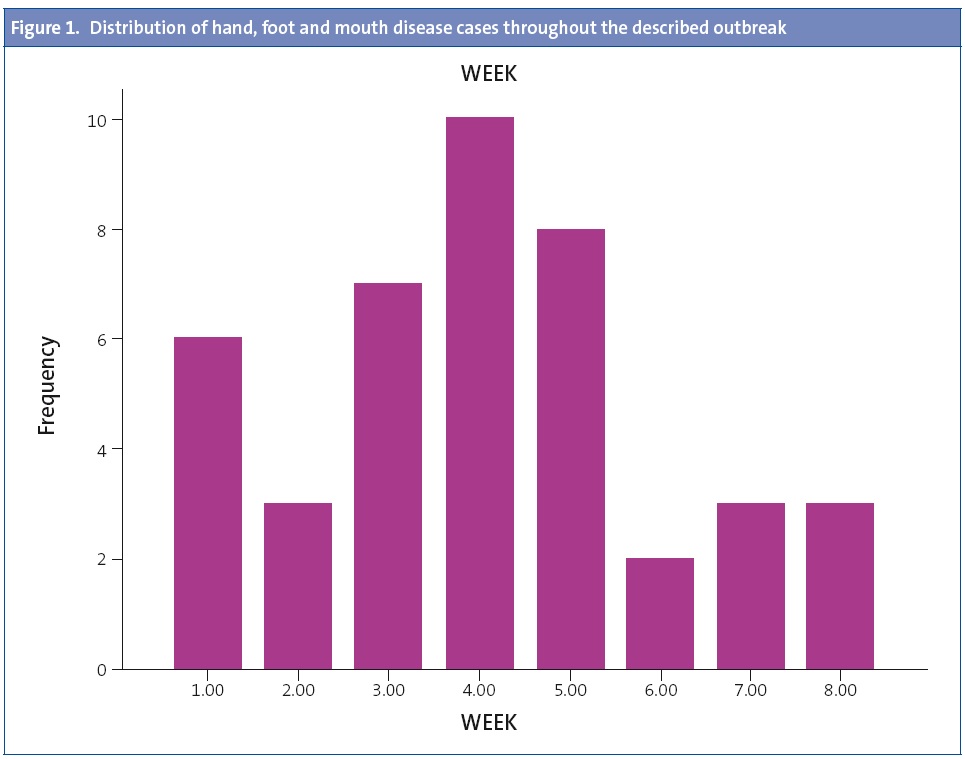
The HFMD outbreak mostly affected children attending child care centres. Most cases had atypical features, such as a predominance of lesions located in the perianal area and the groin (as opposed to the feet), and a predominance of perioral sores over oral ones. Many of the patients affected by the outbreak were diagnosed with onychomadesis within 2 to 3 months after the HFMD episode.
A microbiology study (viral culture from a sample of saliva collected during the acute phase of the disease) was performed in only one patient, leading to the detection of Coxsackie enterovirus. The serotype was not specified.
In the weeks following the HFMD outbreak the incidence of alopecia areata in paediatric patients was higher than usual in our health area (4 cases in the four weeks following the outbreak, compared to 3 cases diagnosed in the 11 months that preceded the outbreak, and no cases the year before).
The mean age of the patients diagnosed with alopecia areata following the HFMD outbreak was 10 years and 9 months (standard deviation, 41.28 months; range, 5 years and 9 months–13 years and 5 months). None of the cases of alopecia areata had been diagnosed with HFMD in the previous months. All cases of alopecia areata were self-limited, with the hairless patches recovering in the ensuing months after treatment with topical steroids or watchful waiting. None of the patients suffered from a nail disorder or an autoimmune pathology.
DISCUSSION
We conducted a clinical-epidemiological observational study. The study has methodological limitations mostly due to the need to identify patients based on the diagnosis made by the clinician. There may have been additional cases of HFMD that did not require medical assistance or were not documented as such in the OMI-AP. Likewise, it is possible that there were more cases of alopecia areata during the study period that were not diagnosed. We could not perform a statistical analysis of the clinical features of the cases (location of skin lesions, presence of oral and perioral lesions, maximum temperature, subsequent development of onychomadesis, etc), as these data were not consistently entered in the medical records, and attendance to child care could not be analysed for the same reason. Furthermore, we only gathered the data for paediatric patients, even though some cases in adult patients were reported during the HFMD outbreak.
Microbiology studies have shown that the coxsackievirus serotypes that caused HFMD most frequently in Spain in 2012 were A6 and A16.22 Although we did not identify the serotype or serotypes involved in the outbreak under study, the atypical features of HFMD and the existence of adult cases lead us to suspect the potential involvement of an emerging serotype, such as coxsackievirus A6.
Despite the aforementioned limitations, the study confirmed that a greater-than-usual number of cases of alopecia areata were diagnosed in the paediatric age group in our health area following the outbreak of HFMD.
The described timeline does not in any way prove causality, as the number of cases is small and microbiology and serology tests were not performed on patients diagnosed with alopecia areata.
Nevertheless, the association between alopecia areata and other autoimmune and immunologic disorders, as well as the association found between enterovirus infections and autoimmune diseases such as type 1 diabetes mellitus, lead us to hypothesise a potential causal relationship between enterovirus infections that cause HFMD and alopecia areata.
The immunologic damage described in the literature consists of perifollicular and intrafollicular lymphocytic infiltrates (CD4+ and CD8+) around hair follicles in the affected areas4 that can react against follicular autoantigens, and altered immune signalling pathways involving several interleukins, IFN- γ, TNF-α and TGF-β.23-25 Similar immunologic alterations have been described in other autoimmune diseases mediated by T-cells in which viral infections may play an important role in the pathogenesis.26
One of the mechanisms proposed to explain the relationship between viral infections and autoimmunity is that a first viral infection could induce immunity against the causative agent (in this case, a virus) but subsequent reinfections (which may cause few symptoms) or repeated exposure to similar agents (for instance, different viral serotypes) could give rise to an abnormal immune response against the host, triggering an autoimmune inflammatory process in specific target organs. This process would only take place in patients with a particular genetic predisposition (associated with polymorphisms in HLA complex, vitamin D receptor, or CTLA4 genes, among others) and could be modulated by additional environmental factors (vitamin D levels, dietary factors, climate factors, other infections, etc).
Such a pathogenic mechanism has been confirmed for dengue haemorrhagic fever, in which the most severe features, despite its viral aetiology, are caused by autoimmune mechanisms triggered by episodes of reinfection,27 and has also been suggested for autoimmune diseases such as type 1 diabetes.28,29
In our study, this reinfection/re-exposure mechanism would explain the development of cases of alopecia areata in older patients (who had probably been infected by enterovirus in the past) following the outbreak of HFMD in preschool-age children. Thus, the increased circulation of enterovirus during the HFMD outbreak would contribute to the higher-than-usual incidence of alopecia areata in the following weeks.
We would also like to suggest the possibility that there is an autoimmune component in cases of onychomadesis associated with HFMD, as nail disorders, including onychomadesis, are often described in association with alopecia areata.30
It would be interesting to tests these hypotheses in future research, possibly by performing microbiology or serology tests in patients recently diagnosed with alopecia areata to establish whether they had had past or recent infections by different enterovirus serotypes.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this paper.
ABBREVIATIONS: HFMD: hand-foot-mouth disease • OMI-AP: primary care electronic medical records database.
BIBLIOGRAPHY
- Cunliffe WJ, Hall R, Stevenson CJ, Weightman, D. Alopecia areata thyroid disease and autoimmunity. Br J Dermatol. 1969;81:877-81.
- Hordinsky M, Ericson M. Autoimmunity: Alopecia areata. J Investig Dermatol Symp Proc. 2004;9:73-8.
- Muller SA, Winkelmann RK. Alopecia areata. Arch Dermatol. 1963;88:290-7.
- Alzolibani AA. Epidemiologic and genetic characteristics of alopecia areata (part 1). Acta Dermatovenerol Alp Panonica Adriat. 2011;20:191-8.
- Dudda-Subramanya R, Alexis AF, Siu K, Sinha AA. Alopecia areata: genetic complexity underlies clinical heterogeneity. Eur J Dermatol. 2007;17:367-74.
- Ito T, Tokura Y. Alopecia areata triggered or exacerbated by swine flu virus infection. Dermatol. 2012;39:863-4.
- Hayderi LE, Nikkels-Tassoudji N, Nikkels AF. Hair loss after varicella zoster virus infection. Case Rep Dermatol. 2013;5:43-7.
- Hyöty H, Hiltunen M, Knip M, Laakkonen M, Vähäsalo P, Karjalainen J, et al. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Childhood Diabetes in Finlan (DiMe) Study Group. Diabetes. 1995;44:652-7.
- Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ. 2011;342:d35.
- Clementz GC, Mancini AJ. Nail matrix arrest following handfoot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Davia JL, Bel PH, Ninet VZ, Bracho MA, González-Candelas F, Salazar A, et al. Onychomadesis outbreak in Valencia, Spain associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Wei SH, Huang YP, Liu MC, Tsou TP, Lin HC, Lin TL. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Fujimoto T, Iizuka S, Enomoto M, Abe K, Yamashita K, Hanaoka N. Hand, foot, and mouth disease caused by coxsackievirus A6, Japan, 2011. Emerg Infect Dis. 2012;18:337-9.
- Osterback R, Vuorinen T, Linna M, Susi P, Hyypia T, Waris M. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-8.
- Wu Y, Yeo A, Phoon MC, Tan EL, Poh CL, Quak SH, The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:e1076-81.
- Bernier V, Labrèze C, Bury F, Taïeb A. Nail matrix arrest in the course of hand, foot and mouth disease. Eur J Pediatr. 2001;160:649-51.
- Bracho MA, Gonzalez-Candelas F, Valero A, Cordoba J, Salazar A. Enterovirus co-infections and onychomadesis after hand, foot, and mouth disease, Spain, 2008. Emerg Infect Dis. 2011;17:2223-31.
- Redondo Granado MJ, Torres Hinojal MC, Izquierdo López B. Brote de onicomadesis posvirica en Valladolid. An Pediatr (Barc). 2009;71:436-9.
- Guimbao J, Rodrigo P, Alberto MJ, Omeñaca M. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15: pii:19663.
- Kaminska K, Martinetti G, Lucchini R, Kaya G, Mainetti C. Coxsackievirus A6 and Hand, Foot and Mouth Disease: Three Case Reports of FamilialChild-to-Immunocompetent Adult Transmission and a Literature Review. Case Rep Dermatol. 2013;5:203-9.
- Haneke E. Onychomadesis and hand, foot and mouth disease, is there a connection? Euro Surveill. 2010;15:pii: 19664.
- Cabrerizo M, Tarragó D, Muñoz-Almagro C, Del Amo E, Domínguez-Gil M, Eiros JM, et al. Molecular epidemiology of enterovirus 71, coxsackievirus A16 and A6 associated with hand, foot and mouth disease in Spain. Clin Microbiol Infect. 2014;20:150-6.
- Bertolini M, Gilhar A, Paus R. Alopecia areata as a model for T cell-dependent autoimmune diseases. Exp Dermatol. 2012;21:477-9.
- Gregoriou S, Papafragkaki D, Kontochristopoulos G, Rallis E, Kalogeromitros D, Rigopoulos D. Cytokines and other mediators in alopecia areata. Mediators Inflamm. 2010:928030.
- Ito T. Recent Advances in the Pathogenesis of Autoimmune Hair Loss Disease Alopecia Areata. Clin Dev Immunol. 2013:348546.
- Pender MP. CD8+ T-Cell Deficiency, Epstein-Barr Virus Infection, Vitamin D Deficiency, and Steps to Autoimmunity: A Unifying Hypothesis. Autoimmune Dis. 2012:189096.
- Malavige GN, Ogg G. Pathogenesis of severe dengue infection. Ceylon Med J. 2012;57:97-100.
- Tracy S, Drescher KM, Chapman NM. Enteroviruses and type 1 diabetes. Diabetes Metab Res Rev. 2011;27:820-3.
- Sarmiento L, Cubas-Dueñas I, Cabrera-Rode E. Evidence of association between type 1 diabetes and exposure to enterovirus in Cuban children and adolescents. MEDICC Rev. 2013;15:29-32.
- Tosti A, Morelli R, Bardazzi F, Peluso AM. Prevalence of nail abnormalities in children with alopecia areata. Pediatr Dermatol. 1994;11:112-5.






