Prevention of serogroup B meningococcal disease using a four-component vaccine
Ángel Gila, D Barrancob, J Batallac, JM Bayasd, M Campinse, Pedro J. Gorrotxategi Gorrotxategif, J Lluchg, Federico Martinón Torresh, M.ª José Mellado Peñai, D Moreno-Pérezj, B Urielk, JA Vázquezl
aFacultad de Ciencias de la Salud. Universidad Rey Juan Carlos. Alcorcón. Madrid. España.
bTécnico de Salud Pública. Madrid. España.
cEspecialista en Medicina Preventiva y Salud Pública. Barcelona. España.
dServicio de Medicina Preventiva. Centro de Vacunación de Adultos. Hospital Clínic. Barcelona. España.
eServicio de Medicina Preventiva y Epidemiología. Hospital Vall d’Hebron. Barcelona. España.
fPediatra. CS Pasaia San Pedro. Pasajes. Guipúzcoa. España.
gEspecialista en Medicina Preventiva y Salud Pública. Valencia. España.
hUnidad de Cuidados Intensivos de Pediatría. Hospital Clínico Universitario de Santiago. Universidad de Santiago de Compostela. Santiago de Compostela. La Coruña. España.
iUnidad de Enfermedades Infecciosas y Tropicales Pediátricas. Servicio de Pediatría. Hospital Carlos III. Madrid. España.
jUnidad de Infectología Pediátrica. Hospital Materno-Infantil Carlos Haya. Málaga. España.
kServicio de Medicina Preventiva. Complexo Hospitalario Universitario de Ourense. Ourense. España.
lLaboratorio de Referencia de Meningococos. Instituto de Salud Carlos III. Madrid. España.
Correspondence: A Gil. E-mail: angel.gil@urjc.es
Reference of this article: Gil A, Barranco D, Batalla J, Bayas JM, Campins M, Gorrotxategi Gorrotxategi PJ, et al. Prevention of serogroup B meningococcal disease using a four-component vaccine. Rev Pediatr Aten Primaria. 2014;16:108.e55-e74.
Published in Internet: 23-06-2014 - Visits: 48844
Abstract
Introduction: meningococcal disease is an infection caused by Neisseria meningitidis, and those of serogroup B are currently the most predominant. It has been difficult to create effective vaccines for this serogroup in order to modify or reduce its morbidity. The aim of this study was to review existing data on the new vaccine 4 CMenB and its potential contribution to the prevention of this infection.
Methods: a panel of 12 experts (from Pediatrics, Public Health and Vaccinology background) conducted a literature search and prioritized 74 publications. A review of the vaccine was then prepared, it was discussed in a meeting and subsequently validated by e-mail.
Results: 4 CMenB vaccine, based on four components (NadA, fHbp, NHBA and OMVnz), was designed by reverse Vaccinology. The Meningococcal Antigen Typing System shows a potential of 70-80% coverage of the strains in Europe. Clinical trials show that the vaccine is safe and immunogenic in infants, children, adolescents, and adults, and induces an anamnestic response. The incidence of fever is similar to systemic vaccines administered alone, but higher when coadministered with them, although the fever pattern is predictable and self-limited.
It is compatible with the Spanish routine vaccines, and can be administered simultaneously with the currently available hexavalent and pentavalent vaccines, as well as the pneumococcal conjugate vaccine.
Conclusions: the 4 CMenB vaccine is the only currently available strategy to prevent meningococcal disease caused by serogroup B.
Keywords
● Meningitis ● Meningococcal meningitis ● Meningococcal vaccines ● Prevention and control ● VaccinesMENINGOCOCCAL DISEASE
Definition and general concepts
Meningococcal disease is a serious infection caused by Neisseria meningitidis (N. meningitidis) that can have several clinical presentations such as meningitis and sepsis.
Twelve serogroups of N. meningitides have been identified, 6 of which (A, B, C, W135, X and Y) can infect humans, although currently there is some controversy surrounding their nomenclature. Most of them are endemic. The epidemiological data and serogroup circulation varies by geographical region, and all types can produce outbreaks.1
The serogroup B strains that cause invasive disease are more genetically diverse than those of other serogroups. Infection by meningococcus B (MenB) is the main cause of invasive disease in developed countries, where infants and adolescents are the populations most vulnerable to the severest forms of the disease.2
Epidemiology and burden of disease
Geographical and seasonal distribution
Most cases of meningococcal disease occur in winter and early spring.2-7 The epidemiology of the disease varies depending on the geographical area and the serogroup.8,9 Serogroup A is responsible for large epidemics in Africa, while groups B and C predominate in developed countries and cause most cases in Europe and the American continent. Serogroup W135 causes epidemics like the one in Saudi Arabia, and in late 2012 it was responsible for a considerable number of cases in the African meningitis belt, Argentina, and Chile.10. Serogroup Y is the most frequent agent of meningococcal disease in the United States and Colombia, and is very common in Canada and Israel. Serogroup Y has caused epidemics in Ghana and a few other African countries.2
The reasons for the uneven distribution of serotypes across the world are not understood, but differences in population immunity and environmental factors play a key role in it.
Meningococcal disease in Europe
According to data from the European Centre for Disease Prevention and Control (ECDC), the incidence of meningococcal disease in Europe ranges from 0.13 to 3.37 cases per 100 000 inhabitants (year 2009)9. The predominant serogroups in Europe in the 1990s were serogroups B and C9. Still, the incidence of invasive meningococcal disease has shown a considerable decline in the past decade due to the introduction of meningococcal C conjugate vaccines in the routine childhood immunisation schedule of many countries4 (from 1.9 per 100 000 inhabitants to 0.92 per 100 000 inhabitants). As a result, serogroup B is the predominant type in Europe now. This serogroup tends to cause epidemic waves subject to long cycles.
The incidence of meningococcal disease varies by age, with the highest rates found in infants, followed by adolescents and young adults. According to 2009 data for Europe, the incidence in infants was of 15.9 cases per 100 000 inhabitants, followed by children aged 1 to 4 years (5.4 per 100 000 inhabitants) and adolescents aged 15 to19 years (2.0 per 100 000 inhabitants)2-4.
Serogroup B causes most cases of meningococcal disease in Europe. Of the reported cases from 2009 for which there was data on the capsular group (amounting to 88%), 71% were caused by serogroup B (particularly in those countries that had introduced conjugated vaccines against serogroup C), 13% by serogroup C, and 4% by serogroup Y. Between 1993 and 1996, serogroup B was already the cause of 68% of reported cases in Europe.2-4,11
As for the distribution of serogroups by age, a large proportion of the cases caused by serogroup B involved young children. The analysis of 4435 cases of invasive meningococcal disease reported in England and Wales over a period of 4 years (2006–2010) showed that 58% of the cases caused by serogroup B occurred in children younger than five years, and that this serogroup was predominant in this age group, accounting for 94% of the cases.4
Meningococcal disease in Spain
In Spain, meningococcal disease is subject to mandatory, urgent, and individual reporting. Cases are registered in the Red Nacional de Vigilancia Epidemiológica (National Network of Epidemiological Surveillance) after being reported to the Sistema de Enfermedades de Declaración Obligatoria (Mandatory Notification Disease System [EDO])12.
According to data published in weekly epidemiological bulletins between 2006 and 2007, the incidence rate of reported cases (confirmed cases and suspected but not confirmed cases) was of 1.37 per 100 000 inhabitants, and decreased to 1.21 per 100 000 inhabitants in 2010.12,13 However, while there have been considerable reductions in the hospitalisation and mortality rates associated to this disease in recent years, morbidity and mortality continue to be significant in children younger than 5 years.14
Incidence rates vary across the various autonomous communities in Spain. In the 2006–2007 period, some autonomous communities had incidence rates of up to 4.19 cases per 100 000 inhabitants, and in 2009–2010 some reached rates of up to 3.33 per 100 000 inhabitants.12,13Table 1 shows the incidence rates for 201012.
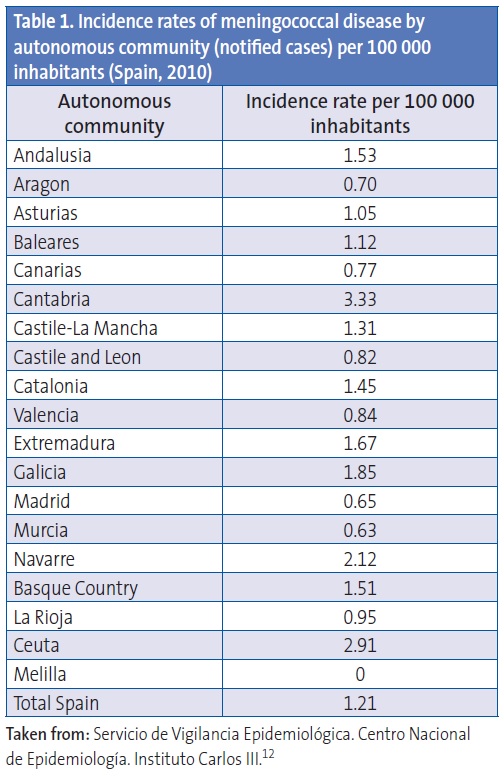
The introduction of the vaccine against serogroup C in 2000 brought on a significant change in the epidemiology of meningococcal disease in Spain, with serogroup B becoming the main causative agent of invasive disease. Since there is no effective vaccine against serogroup B, it is difficult to change or reduce the morbidity and mortality associated with meningococcal disease in Spain. The incidence rates of confirmed cases in recent years (approximately from 2006 to 2010) have ranged between 0.17 and 0.12 for serogroup C and 1.12 and 0.69 for serogroup B. The decline observed in the number of cases caused by serogroup B, particularly in 2010, is similar to that observed in other European countries9. This decline may be due to the cyclical nature of the disease2-6 and be determined by various environmental factors and risk behaviours.2 The cyclical pattern of the disease requires strict surveillance to obtain the necessary data for the potential use of vaccines with different formulations, and to monitor the impact of using these vaccines. For example, in the decade of 1975 to 1985, meningococcal disease reached rates of 30 cases per 100 000 inhabitants in the autonomous community of Galicia.15 Only 10 to 30 cases caused by other serogroups (A, W135, and Y) are reported in Spain, depending on the season, with incidence rates ranging from 0.02 to 0.06 per 100 000 inhabitants).12,13
Consequences of meningococcal disease
It is estimated that 10% to 14% of meningococcal disease cases are fatal, and that 8% to 20% of survivors have long-term neurological sequelae.16 Some of the sequelae associated to bacterial meningitis and meningococcal septicaemia are hearing loss, amputations, skin complications, psychosocial impairments, hydrocephalus, neurological and developmental impairments, and kidney failure.16-19
It is important that we study the economic impact and the burden of hospitalisation associated to meningococcal disease in Spain. A study on the hospitalisations and fatalities associated with meningococcal disease based on CMBD data from 1997 to 2008 found an annual hospitalisation rate of 2.33 per 100 000 inhabitants14, with an associated cost of more than 5 million euro per year.
Mode of transmission and clinical manifestations
Carriage and transmission of meningococcal disease
Humans are the only known reservoir of N. meningitidis, and the upper respiratory tract is the main locus of infection. The presence of meningococcus in the upper respiratory tract can be transient; lead to colonisation (carriage); or produce invasive disease.16
Meningococcus is transmitted from person to person through the secretions of the upper respiratory tract of asymptomatic carriers or diseased individuals.16,20 The latency period usually lasts 3 to 4 days, but it may range from 2 to 7 days. Individuals that do not develop disease in the 7 days following colonisation may remain asymptomatic carriers.21 Although most studies on asymptomatic carriage are cross-sectional, some studies with longitudinal nasopharyngeal samples have concluded that carrier state may be chronic, lasting for several months; intermittent (with consecutive colonisation by different strains); or simply transient, lasting a few days or weeks.22
The prevalence of carriers in the general population varies widely, ranging from 0.6% and 34.4% according to several studies.23-27 The carriage rate is greater among individuals that dwell in closed settings, such as childcare centres, schools, universities, dormitories, or military barracks; in active or passive smokers; and in individuals with diseases of the upper respiratory tract.16
Clinical manifestations
Sepsis and meningitis are the two most common forms of meningococcal disease. They may develop in isolation or simultaneously in the same patient. Meningococcal septicaemia presents with fever, petechiae, and maculopapular rash.28 In most cases, the skin lesions appear in the first 24 hours following onset of fever.28 Between 10% and 20% of patients develop fulminant sepsis, characterised by purpura, hypotension, myocardial dysfunction, and finally multiorgan failure, with a high mortality rate.28
Meningitis without sepsis typically presents with vomiting, photophobia, headache, stiff neck, altered level of consciousness ranging from obtundation to coma, and in infants, a bulging fontanel or refusal to feed. Skin lesions are rare in meningitis without sepsis.29
It is important to take into account that symptoms of meningococcal disease, with or without sepsis, may be nonspecific in the early hours and can be mistaken for signs of a viral infection.17,30
In rare cases, meningococcal infection can cause other conditions, such as arthritis, pneumonia, endocarditis, or pericarditis.29
Diagnosis
Early diagnosis of meningococcaemia is particularly challenging and requires a high level of clinical suspicion and microbiological confirmation.16,31
The most sensitive testing method is the polymerase chain reaction (PCR), whose results are not affected by prior treatment with antibiotics. PCR methods are also used to confirm the genotype (serogroup) and subgenotypes (serosubtypes). Real time PCR is the most frequently used technique.
Still, bacterial culture of a bodily fluid that is sterile under normal conditions, such as blood or cerebrospinal fluid (CSF), or the Gram stain procedure for CSF, continue to be the most widely used methods in hospital settings. It is important to note that the sensitivity of these methods declines considerably after initiation of antibiotic therapy.3,16
Nonculture methods, such as the use of commercially available kits to detect polysaccharide antigen in the CSF, have been developed to facilitate and enhance laboratory diagnosis. These methods are rapid and specific and can provide a serogroup-specific diagnosis, but false negative results are common, and there can be cross-reactivity with other serogroups, especially in infection by serogroup B3. Consequently, these methods are not usually included among those accepted for confirmation of a case.
Isolation of the bacterium from nasopharyngeal swabs is not sufficient for diagnosis. It is only indicative of colonisation, and therefore its use is not recommended for diagnosis of invasive disease.16
Treatment
Meningococcal disease is potentially fatal and must always be considered a medical emergency.32 Early antibiotic therapy is one of the most important factors in the prognosis of the disease, so treatment should be initiated during the visit to the healthcare centre, before the patient is referred to the hospital.
The treatment of choice is cefotaxime or ceftriaxone until antimicrobial susceptibility testing results are available.16,32
During hospitalisation, the only precaution necessary is preventing contact with respiratory secretions of the patient (droplet isolation) for the first 24 hours of antibiotic treatment.
Prevention
Chemoprophylaxis
There are two types of transmission sources: the asymptomatic carrier and the symptomatic patient. Secondary cases of disease can be prevented by eradication of the carrier status of those individuals likely to have colonisation of the upper respiratory tract, such as contacts in a nursery or school, or the household members of an index patient.31
The risk of contracting the disease from contact with a patient is highest in the first days of the disease (from a week prior to onset of symptoms to 24 hours after the index patient starts the appropriate antibiotic treatment).3,16 The attack rate of meningococcal disease in individuals in close contact with the patient has been estimated to be between 400 and 800 times greater than in the general population. In these cases, nasopharyngeal cultures are not useful to determine who needs chemoprophylaxis, and is thus not recommended.33
The purpose of chemoprophylaxis is to lower the risk of acquiring invasive disease by eradicating carrier status in contact groups. Chemoprophylaxis succeeds in reducing the risk of contracting the disease by more than 80%. The antibiotics administered as chemoprophylaxis are meant to eradicate nasopharyngeal carrier status and must be administered as soon as possible, as they are likely to be of little or no benefit if given more than 14 days after the onset of disease in the index patient.3,16
Two of the antibiotics most recommended and widely used in clinical practise are rifampicin and ciprofloxacin. The efficacy of ofloxacin, azithromycin, and ceftriaxone has also been demonstrated, although the usefulness of azithromycin is debated due to the observed bacterial resistance.16,31
Since secondary cases may appear several weeks after contact with the index case, vaccination against meningococcus can be a very useful complement to prophylaxis when an outbreak is caused by a serogroup for which there is an available vaccine.33 However, mass chemoprophylaxis programmes are not recommended to control large outbreaks of meningococcal disease. This approach is impractical and unlikely to be successful due to several factors, such as the multiple sources of infection, the prolonged risk of exposure, logistic problems, and high cost.3
Vaccination
The most effective preventive strategy to control meningococcal disease is vaccination.3,34 To have optimal impact on prevention of this disease, the vaccines must be included for administration at an early age in childhood immunisation schedules.4
Types of meningococcal vaccines
Unconjugaged polysaccharide vaccines. The earliest effective meningococcal vaccines based on purified capsule polysaccharide were developed in the 1960s against serogroups A and C, followed by similar vaccines against serogroups Y and W135 in the 1980s. These vaccines have played a prominent role in disease prevention for decades, but have significant limitations35: they are not immunogenic in infants, they do not induce immunological memory, and they do not confer mucosal protection, and thus cannot induce herd immunity.34,36 The development of conjugate vaccines against these serogroups has been an essential step in establishing long-term protection. However, it was not possible to develop a vaccine against serogroup B using its capsule polysaccharides.
Conjugate vaccines. Glycoconjugate vaccines against serogroup C were developed in the 1990s. Since 1999, they have been introduced in several European countries (first in the United Kingdom and Spain, then gradually in others), Australia, United States, and Canada. Conjugation was able to overcome the limitations of polysaccharide vaccines.
Monovalent formulations against serogroup C were followed by multivalent glycoconjugate formulations, and in 2005 the first glycoconjugate vaccine against serogroups A, C, Y and W135 was licensed for use in the United States. Currently, there exist 3 quadrivalent conjugate vaccines against serogroups A, C, Y and W135, which differ in their transport protein component,34,37 although only 2 of them are currently available in Spain, requiring prescription and restricted to hospital use.
A glycoconjugate vaccine against serogroup A has been available since December 2010. The vaccine aims to control disease caused by this serogroup, whose rate of incidence in the African meningitis belt is high.34,37
This vaccine came from a novel experience combining the efforts of international organisations such as the WHO and of corporate enterprise. The vaccine can help control this disease in a region where poverty and limited resources pose barriers to the solution of major public health problems, as is the case of meningitis caused by group A meningococcus.
The use of meningococcal conjugate vaccines has been a key step in the prevention of the disease. However, meningitis caused by serogroup B meningococcus has yet to be controlled.
Effectiveness of vaccination strategies. Throughout time, different vaccination strategies have proven to be effective in controlling meningococcal infection. In the early 1990s there was an increase in the incidence of invasive cases by serogroup C in Europe. After the introduction of conjugate vaccines against this serogroup in the routine immunisation programmes of several European countries, the incidence of disease caused by serogroup C dropped dramatically, and the vaccine had proved its ability to induce herd immunity. The postvaccination reduction in serogroup C has led to the current predominance of serogroup B.9,36,38
Specific data from European countries, such as the United Kingdom, the Netherlands, or Spain, demonstrate this fact. In the United Kingdom, the use of the conjugate vaccine against meningococcus C (introduced in its routine childhood immunisation schedule in 1999, including a catch-up dose to be given at up to 18 years of age) was associated with a significant and sustained reduction of invasive meningococcal disease caused by this serogroup, with rates of only 0.02 per 100 000 inhabitants (a reduction from 955 cases to 13) in the 2008–2009 period.36,38
In the Netherlands, the conjugate vaccine against meningococcus C was introduced in the routine immunisation schedule in 2002 with a single dose at 14 months. The same vaccine was used to implement a national catch-up campaign in children 1 to18 years of age, with a coverage of approximately 94%.39
The vaccine was introduced in the Spanish routine immunisation schedules in 2000 to be administered at 2, 4, and 6 months of age, along with a catch-up programme for children younger than 6 years that was later expanded to age 18 years, although the schedule varied widely between the country’s various autonomous communities and depending on the year it was introduced. The incidence of disease in infants and children aged up to 9 years declined from 7.04 cases per 100 000 inhabitants (1999–2000) to 1.08 per 100 000.36 The effectiveness of the programme has been 95.2% for infants and 97.8% for catch-up immunisation of children younger than 6 years.40 Population protection has been less pronounced than in other countries, since the catch-up campaign initially targeted children younger than 6 years and did not include adolescents, the age group with the highest rate of carriers.40 While a vaccine effectiveness of 94% was observed 4 years after the introduction of the conjugate vaccine in the Spanish immunisation schedule, a loss in protection was also detected in children who had been vaccinated but had not received a booster dose at 12 months of age.40 This led to the modification of the original schedule, postponing the administration of the third dose to the second year of life.40
Evolution of meningitis cases by serogroups in Spain following routine vaccination against serogroup C. A significant drop in infection by serogroup C was observed after the introduction of the conjugate vaccine (year 2000), while the incidence of disease caused by serogroup B had not declined and was actually rising (Figure 1).41 In a ten-year period, there has been an 88% decline in the incidence rate of meningitis C (1999–2000 compared with 2009–2010)12. The same is true of hospitalisation and mortality rates, which have experienced a considerable drop.14 However, the overall reduction in number of cases is lower than the one observed in the United Kingdom or the Netherlands,9 probably due to the low coverage of adolescents in catch-up campaigns in some of Spain’s autonomous communities.
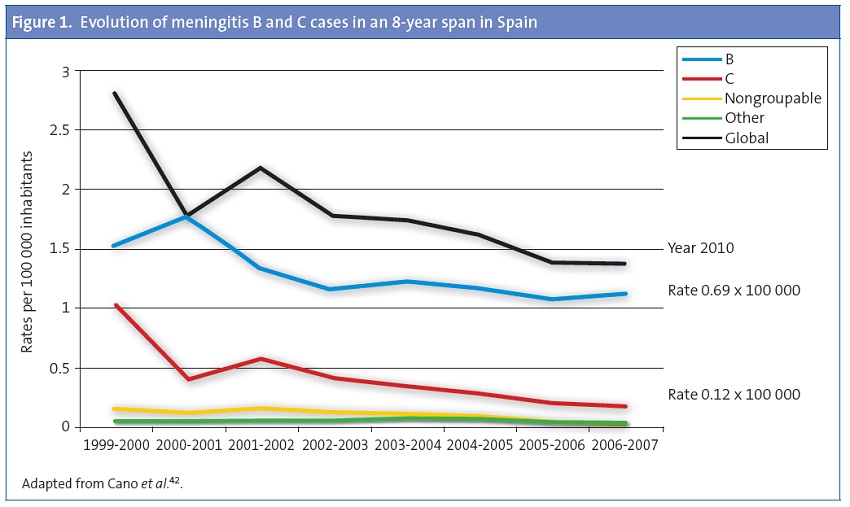
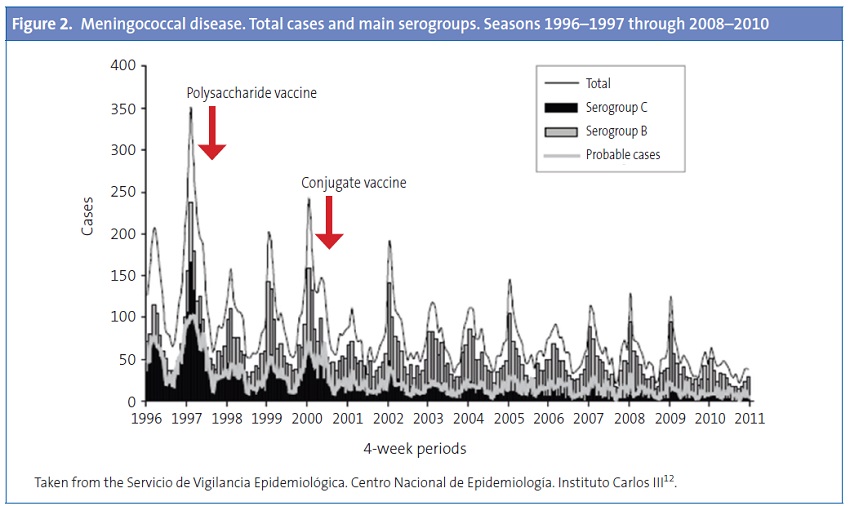
Figure 2also shows that the introduction of the conjugate vaccine has resulted in a decrease in cases caused by serogroup C, while the changes in disease caused by serogroup B have been minor.12
Vaccines against serogroup B
The capsule polysaccharide of serogroup B has a high antigenic similarity with saccharides of human neural tissue and is poorly immunogenic in humans. The use of serogroup B meningococcus polysaccharides for the development of vaccines has been limited by the theoretical risk that these vaccines could overcome immunologic tolerance and induce autoimmunity. Consequently, strategies for developing vaccines against serogroup B disease have focused primarily on noncapsular antigens.3,43
Outer membrane vesicle vaccines. Early attempts to develop a vaccine against serogroup B N. meningitidis were based on outer membrane vesicles (OMVs), which contain several immunogenic antigens, including lipo-oligosaccharide and porin A (PorA). However, lipo-oligosaccharide is an endotoxin that can be harmful to the host, and while attempts have been made to remove it by treatment with detergents, it cannot be eliminated completely and results in a higher reactogenicity. Consequently, lipo-oligosaccharide is no longer included in the formulation of OMV vaccines, and PorA is now their main antigen.44
While PorA in OMV vaccines induces a robust immune response, it is highly variable in serogroup B strains.45 Therefore, vaccines based on this component produce a strain-specific immune response. They are also not sufficiently immunogenic in infants.34,44,46.
At present, OMV vaccines are the only vaccines available to control outbreaks of specific hypervirulent strains of MenB, and they only confer short-term protection. These vaccines are only effective in outbreaks caused by a strain that expresses the specific PorA contained in the vaccine.
OMV vaccines against MenB have been developed and used in Cuba (routinely for 20 years), Norway (in controlled clinical trials), New Zealand (during an outbreak), 47 recently in Normandy (France),48 and years ago in Brazil.49 This limits their use in regions like North American or Europe, where serogroup B disease is caused by a wide range of serosubtypes.36,37,44,45,50,51
Studies with OMV vaccines show that a vaccine with multiple antigens is more likely to cover a larger number of N. meningitides strains, facilitating an immune response against a greater number of different strains, and thus greater protection against meningococcal disease.44
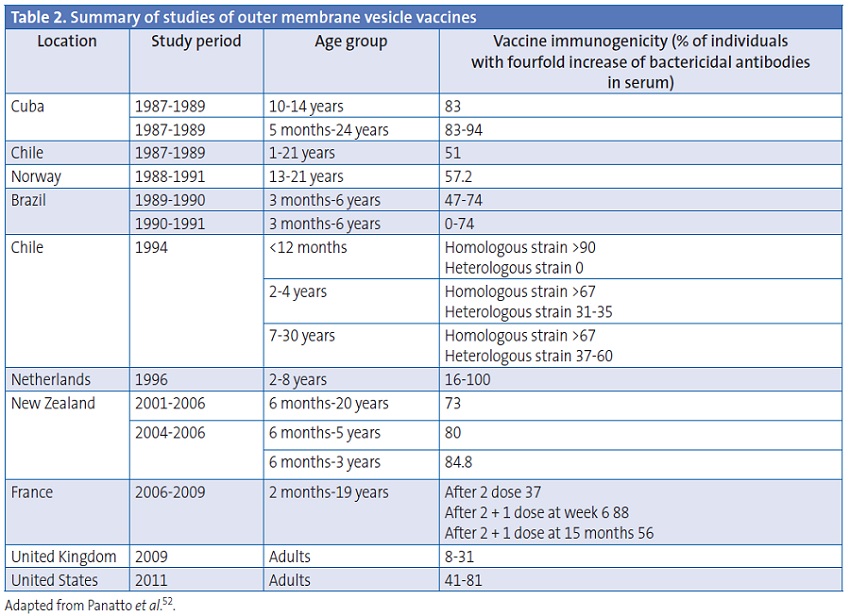
Table 2 shows a summary of the studies done with OMV vaccines.52
Latest-generation vaccines. The considerable diversity of outer-membrane proteins that cause serogroup B disease, as well as geographic and possibly temporal variations, may limit the usefulness of noncapsular antigen vaccines.3 As we saw above, development of a vaccine against serogroup B has been based on candidate antigens, but this approach has yielded few results.34
rLP2086 vaccine. Currently, vaccines based on Factor H binding proteins (fHbps) are being investigated. These proteins segregate into two subfamilies, designated A and B. Vaccines can be monovalent or bivalent depending on whether they contain one or both subfamilies of the protein. It has been observed that the bivalent vaccine (currently being developed by Pfizer),53 composed by both types of fHbp, elicited greater bactericidal activity against the MenB strains expressing these types of fHbp than monovalent vaccines. Bivalent rabbit immune sera in serum bactericidal antibody assays (SBAs) against several strains of MenB killed 87 of the 100 tested isolates. Bivalent human immune sera killed 36 of 45 MenB isolates. The best predictor for killing in the SBA was the level of in vitro surface expression of fHBP.34,54 The bivalent vaccine is currently undergoing phase III clinical trials.
4CMenB vaccine. Novartis Vaccines and Diagnostics has developed a new vaccine against serogroup B based on four components, to which we devote a specific section due to its novel character and its upcoming availability.
THE FOUR-COMPONENT VACCINE AGAINST MENINGOCOCCUS B: A NEW VACCINE APPROACH AGAINST SEROGROUP B MENINGOCOCCUS
Reverse vaccinology
Traditionally, vaccine development has been based on laboratory culture of organisms for the isolation and potential manipulation of their components. Once components are isolated, they are tested for their ability to elicit an immune response. Identifying antigen candidates for vaccine development by this method is very time consuming, and the method cannot be used to develop vaccines against pathogens that lack immunodominant antigens, such as capsular antigens or toxins.
Reverse vaccinology starts from the genomic sequence of the microorganism. Specialised bioinformatic software is used to analyse the open reading frames (ORFs) in the sequence, that is, known genes and DNA fragments that may encode different types of surface proteins in the bacterium (even if their function, regulation, etc. are unknown), determining the regions of the genome that may encode certain functions. This allows the identification of antigens that are most likely to be vaccine candidates. Vaccines are prepared with the selected antigens, and then tested in animal models before the development of the final product.55,56
The reverse vaccinology approach has the advantage that the genome of the microorganism offers a catalogue of virtually all the proteins that the pathogen can express at any given time, whether they are expressed in vivo or in vitro. This facilitates the selection of the proteins that may be surface-exposed starting from the genome, and not the microorganism.55 Despite having some limitations (the inability to identify non-protein antigens, such as polysaccharides or glycolipids56,57), it has been a momentous breakthrough in the development of protein-based vaccines against complex microorganisms.
Design of the four-component vaccine against meningococcus B by reverse vaccinology
The development of a serogroup B meningococcal vaccine constitutes the first example of the successful application reverse vaccinology.56,57 Development started by screening the genome of one MenB strain, which led to the identification of around 600 ORFs. All these ORFs were amplified by PCR and cloned in Escherichia coli. A total of 350 recombinant proteins were expressed, purified, and used to immunise mice.34,55,58,59
The sera obtained from the mice were then tested with several assays: Western blot to confirm that each protein was expressed in vivo and localised in the outer membrane; enzyme-linked immunosorbentassay (ELISA) and fluorescence-activated cell sorteranalysis were performed to verify the surface-localisation of the expressed proteins. Finally, the sera were tested for bactericidal activity, which is a correlate for protection in humans. The immunogenicity analysis identified 28 protein antigens that could induce bactericidal antibodies in serum.34,,55,58,59
Of these 28 antigens, a few were highly conserved in a group of MenB strains and were immunoreactive to sera from convalescent patients with meningococcal disease. The selected antigens were prioritised based on their ability to induce broad protection as inferred by SBA or observed in passive protection in the infant rat or mouse protection assays. The three antigens that met these criteria were the neisserial heparin-binding antigen (NHBA), and genome-derived neisserial antigens: GNA2132, GNA1870 or fHbp, and GNA1994 or neisserial adhesin A (NadA). Two additional antigens, GNA1030 and GNA2091, were included because they induced protective immunity in some assays, and fused to NHBA and fHbp, respectively. Still, none of them induced a broad enough protective response to cover all tested strains. Since the combination of several antigens in a single vaccine confers enhanced protection, the key antigens were combined into a multicomponent vaccine.60Figure 3 shows the process of vaccine development.
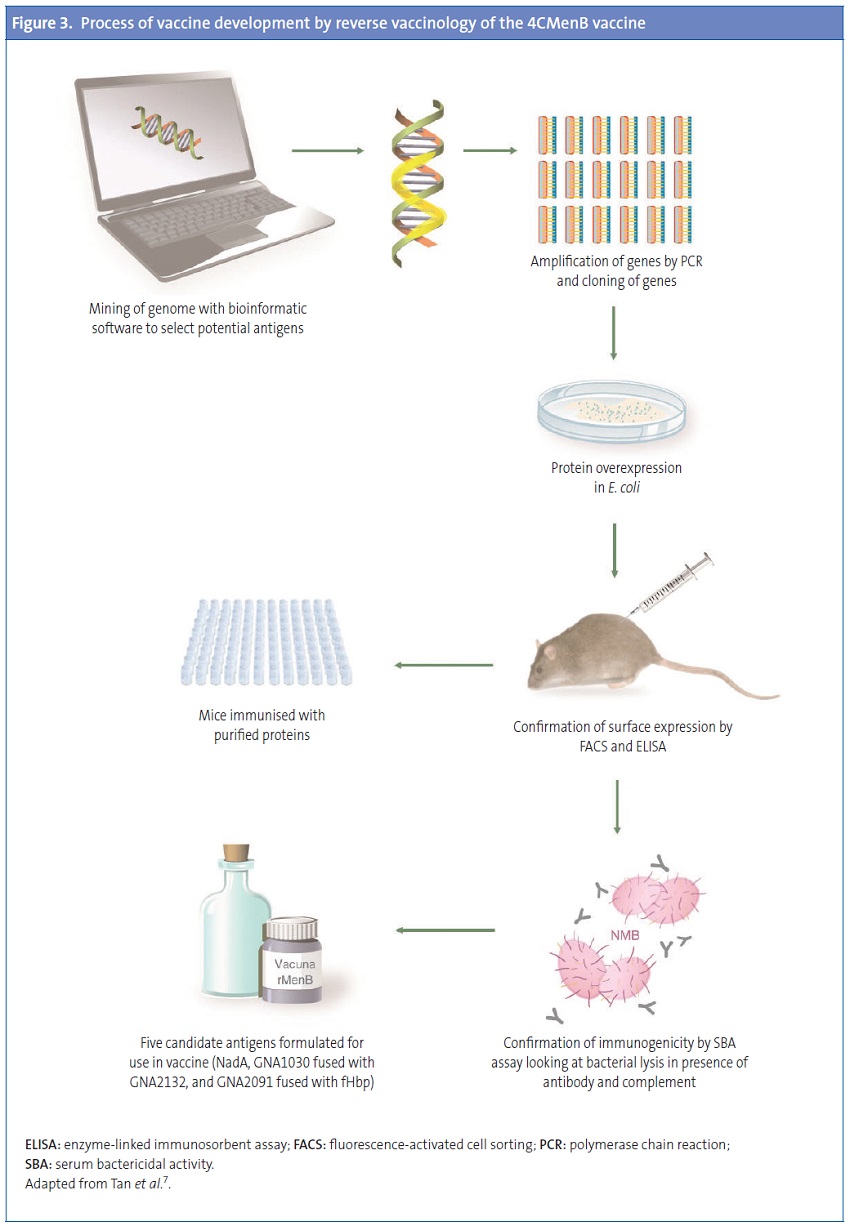
Vaccine components (antigens) and doses of each component
Originally the vaccine was formulated with 3 components—the antigens fHbp, NadA, and NHBA—and called recombinant MenB vaccine (rMenB). Later on, OMVs derived from a Norwegian strain (OMVnw) were added to the formulation, and some trials were conducted with this combination (rMenB + OMVnw). Finally, the Norwegian OMVs were replaced by New Zealand strain OMVs, which offer broader coverage, giving rise to the rMenB + OMVnz vaccine, also known as the 4CMenB in reference to its four components.34
The vaccine is administered by intramuscular injection in 0.5 mL doses. Its composition consists of34,50,61:
- 50 µg recombinant Neisseria meningitidis B NHBA-GNA1030 fusion protein.
- 50 µg recombinant Neisseria meningitidis B NadA protein.
- 25 µg OMV from Neisseria meningitidis B strain NZ98/254, measured as the amount of protein contained by PorA P1.4.
The NadA, GNA2091-fHbp, and NHBA-GNA1030 components are adsorbed onto 1.5 mg of aluminium hydroxide, 3.25 mg of sodium chloride, and 10 mM of histidine.
The antigens included in the vaccine perform the following functions in the bacterium34:
- NadA: there are 5 known variants (Nad1-Nad5). Its function is to promote adhesion and invasion of host epithelial cells, so it may play an important role in carriers of the disease. It also binds to monocyte-derived dendritic cells and macrophages, which may enhance the immune response to NadA following its ingestion and presentation to lymphocytes. Thus, the generation of specific antibodies against this protein could interfere with colonisation, and thus prevent carrier status, although the real impact on carriage remains unknown.
- fHbp: it is an N. meningitidis surface lipoprotein and it is classified into 3 variants (1–3) according to some authors, and into 2 subfamilies (A for variants 2 and 3, and B for variant 1) according to others. This lipoprotein binds the host complement alternative pathway inhibitor (Factor H), helping the bacterium evade complement-mediated killing and increasing its survival. It also binds the bacterial siderophore enterobactin in vitro. Blocking this lipoprotein can help the immune system kill the microorganism.
- NHBA: it is a surface-exposed lipoprotein of N. meningitidis that is a target of both meningococcal and human proteases and binds to heparin in vitro. In the absence of the bacterial capsule, binding to heparin is associated to increased survival of N. meningitidis in human serum, and might facilitate adherence to host tissues. However, we still know little about this antigen.
- OMVnz: the outer membrane vesicles come from a New Zealand epidemic strain, NZ 98/254 (B:4:P1.7-2,4), and were used in the development of vaccines to control an outbreak in this country.62 However, its protection is highly specific, as it mainly confers immunity against its immunodominant antigen, PorA, which is highly variable. Its inclusion in the 4 CMenB vaccine increases its immunogenicity, and offers protection against the strains that express PorA subtype P1.4.
In order to evaluate the immunogenicity of each protein component of the 4CMenB vaccine, clinical trials have used specific strains that express only one of the antigens that compose the vaccine, so that the response to each of the four components could be assessed separately. The strains used in clinical trials have been 44/76-SL (fHbp response), NZ98/254 (PorA response), 5/99 (NadA response), and M10713 (NHBA response). The last one was identified later than the others and has only been analysed in recent studies.
Clinical development and compatibility with other vaccines
Tables 3 and 4 (source available at http://dx.doi.org/10.1016/j.anpedi.2013.04.013) present the available information on the clinical trials of the 4CMenB vaccine, and their main results.34
The clinical development of the vaccine has shown that it is both safe and immunogenic in children as well as adults. It induces immunologic memory and it is compatible with routinely used vaccines. In regards to safety, the most commonly observed local and systemic reactions in infants and children younger than 2 years were pain upon applying pressure and erythema at the site of injection, fever, and irritability. In clinical trials in infants, fever developed more frequently when 4CMenB was administered concomitantly with routine vaccines than when it was administered alone. When 4CMenB was administered alone, the frequency of fever was similar to the frequency of fever associated to routine vaccines administered during the clinical trials. When fever developed, it usually followed a predictable and self-limiting pattern (onset at 6 hours, peak at day 2, end at day 3) and was clinically unimportant. Fever could be prevented by prophylactic administration of paracetamol.
Vaccine coverage (Meningococcal Antigen Typing System)
The necessary steps to evaluate the potential impact of the 4CMenB vaccine are:
- Demonstrating its immunogenicity by means of SBAs using human complement (hSBA) and calculating its protection rate based on the accepted surrogate markers of protection.
- Calculating the strain coverage (proportion of circulating disease-causing strains in a region or country that are potentially killed by immune serum produced by the vaccine). The potential coverage of a vaccine against serogroup B depends on this calculation.
Up to now, the efficacy of vaccines against diseases like MenB, with low incidence rates, that preclude conducting efficacy clinical trials that require the enrolment of a large number of subjects, has been estimated by means of surrogate protection parameters. In the case of meningococcal disease, efficacy has been inferred from SBA results. Serum bactericidal antibody assays have been used to demonstrate the immunogenicity of this new vaccine against serogroup B, testing the four strains that were the source of the different vaccine antigens against sera from immunised individuals.60 Still, conducting clinical trials to assess the overall protective efficacy of this vaccine, as has been done with conjugate polysaccharide vaccines, is not a viable option. In the evaluation of polysaccharide vaccines, the antigen is common to all strains of the serogroup, so sera are tested against a single strain that expresses the antigen. In the case of the four-component vaccine against serogroup B, each serum sample would have to be tested against hundreds of strains expressing all or most of the possible antigenic variants of the serogroup, which is obviously not feasible.
The Meningococcal Antigen Typing System (MATS) is a standardised and reproducible method developed to assess the potential strain coverage of the 4 CMenB vaccine against specific meningococcal strains susceptible to be killed by vaccine-induced antibodies,71 although it could be used in other vaccines.
The MATS uses a vaccine-antigen specific enzyme immunoassay (ELISA) that detects qualitative and quantitative differences in antigen expression; thus, it measures both immunologic cross-reactivity and the quantity of expressed NHBA, NadA, and fHbp antigens. It also includes PorA genotyping information to assess the potential coverage with this antigen. The results obtained using ELISA correlated with the killing of strains in SBAs, and it was found that isolates exceeding a threshold value (positive bactericidal threshold [PBT]) in the ELISA for any of the three vaccine antigens had a ≥80% probability of being killed by immune serum in the SBA. Strains positive for two or more antigens had a greater probability (96%) of being killed in the presence of sera from immunised individuals. The MATS assay allows typing of large panels of strains and prediction of the potential coverage of the vaccine.
Thus, the vaccine’s strain coverage is defined as the proportion of circulating strains that exceed the PBT for at least one vaccine antigen (NadA, NHBA, or fHbp) in the MATS or are matched to the PorA subserotype of the OMV component of the vaccine (P1.4).
The MATS can be used to monitor possible changes in the endemic population of MenB over time, monitoring the distribution of vaccine antigens in isolates of MenB carriers and individuals with MenB disease following the introduction of the vaccine, and to detect the possible emergence of variants resistant to the vaccine.71,72 Using MATS, a study that tested a panel of 1052 MenB strains from 5 European countries (Germany, France, England and Wales, Italy, and Norway [EU5]) predicted that the 4CMenB vaccine would cover 73% to 87% of the strains.73 The data obtained from a similar study in Spain74 showed a potential strain coverage of 69%, only slightly lower than the one predicted by the EU5 study. This difference may be due to a different distribution of meningococcal clonal lineages associated to clinical cases in Spain.
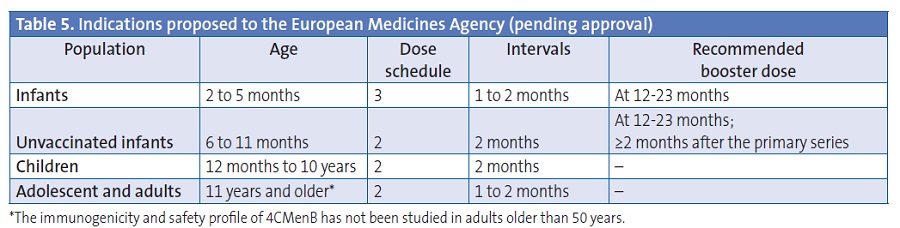
RECOMMENDED VACCINATION STRATEGY: POSOLOGY PROPOSED TO THE EUROPEAN MEDICINES AGENCY
Table 5 presents the posology proposed to the European Medicines Agency.
CONCLUSIONS
The main conclusions of the working group on the consensus document for the 4CMenB vaccine are the following:
- The 4CMenB vaccine is safe and immunogenic in infants, children, adolescents, and adults, and it induces immunological memory.
- The systemic reactogenicity of the 4CMenB vaccine (incidence of fever) is greater than that of routine vaccines when it is administered concomitantly with the latter (although its reactogenicity is similar to that of routine vaccines when administered alone), and the pattern of fever is predictable and self-limiting. The 4CMenB vaccine is compatible with most routine vaccines included in the Spanish immunisation schedule, and it can be administered concomitantly with the currently available hexavalent and pentavalent vaccines, as well as with the heptavalent pneumococcal conjugate vaccine. There are still no data on the concomitant administration with the meningococcal serogroup C vaccine and broad-spectrum pneumococcal vaccines.
- The new 4CMenB vaccine is estimated to cover between 70% and 80% of the circulating strains in Europe.
- At present, the 4CMenB vaccine is the only available strategy to prevent meningococcal disease caused by serogroup B.
CONFLICTS OF INTEREST
D. Barranco D and P. Gorrotxategi have no conflicts of interest to declare in relation to the preparation and publication of this paper.
A. Gil declares having participated in educational and research projects funded by Sanofi, Pfizer, Crucell, GSK, and Novartis.
J. Batalla has received financial compensation from Novartis for participating in a session about meningitis B as an educator.
J. M. Bayas has received professional pay for conferences, consultation, and participation in working groups funded by GSK, Sanofi Pasteur MSD, Laboratorios Esteve, Novartis, and Pfizer. He has also been the principal investigator in clinical trials sponsored by GSK and Sanofi Pasteur MSD.
M. Campins declares having participated in educational and research projects funded by Sanofi, Pfizer, Crucell, GSK, and Novartis.
J. Lluch declares having participated in educational and research projects funded by Sanofi, Pfizer, Crucell, GSK, and Novartis.
F. Martinón-Torres develops his research activity with funding from the Instituto de Salud Carlos III (Intensificación de la actividad investigadora and FISPI1000540) from the national R&D plan and the FEDER funds.
M. J. Mellado has received funding to impart vaccine update courses for GSK in 2006–2007, impart a course on vaccines for the Asociación Española de Pediatría (Spanish Association of Paediatrics) funded by Sanofi Pasteur MSD, and to participate in the Foro de Vacunación Pediátrico de Rotavirus y Varicela 2011.
D. Moreno-Pérez has collaborated in educational activities funded by GSK, Novartis, Pfizer, and Sanofi Pasteur MSD, as a researcher in GSK clinical trials, and as a consultant in the Advisory Board of Pfizer and Astra-Zeneca.
B. Uriel has provided consultation for Novartis.
J. A. Vázquez declares that the laboratory of which he is director has received and continues to receive funding from Baxter, Novartis, Pfizer, Sanofi-Pasteur, GSK, and Laboratorios Esteve through different collaboration agreements.
COLLABORATION
This work has been done in collaboration with the laboratories of Novartis Vaccines and Diagnostics.
ACRONYMS: ECDC: European Centre for Disease Prevention and Control • EDO: Enfermedades de Declaración Obligatoria (Mandatory Notification Disease) • ELISA: enzyme-linked immunosorbent assay • fHbp: factor H binding protein • GNA: genome-derived neisserial antigens • CSF: cerebrospinal fluid • MATS: Meningococcal Antigen Typing System • MenB: meningococcus B • NadA: Neisserial adhesin A • NHBA: Neisserial heparin-binding antigen • OMV: Outer Membrane Vehicle • ORF: Open Reading Frames • PBT: Positive Bactericidal Threshold • PCR: polymerase chain reaction • PorA: porin A • rMenB: recombinant meningococcal group B vaccine • SBA: serum bactericidal activity.
BIBLIOGRAPHY
- World Health Organization. Meningococcal meningitis factsheet. December 2011 [on line] [consulted on 18/09/2012]. Available in: http://www.who.int/mediacentre/factsheets/fs141/en/index.html
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27 Suppl 2:B51-63.
- Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344:1378-88.
- Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, Ladhani SN, et al. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2011;30 Suppl 2:B26-36.
- Schwartz B, Moore PS, Broome CV. Global epidemiology of meningococcal disease. Clin Microbiol Rev. 1989;2 Suppl:S118-24.
- Al-Tawfiq JA, Clark TA, Memish ZA. Meningococcal disease: The organism, clinical presentation, and worldwide epidemiology. J Travel Med. 2010;17 Suppl:3-8.
- Tan LK, Carlone GM, Borrow R. Advances in the development of vaccines against Neisseria meningitidis. N Engl J Med. 2010;362:1511-20.
- World Health Organization. Meningococcal vaccines: WHO position paper, November 2011. Weekly epidemiological record. 2011;47:521-40 [on line]. Available in: http://www.who.int/wer/2011/wer8647.pdf
- European Centre for Disease Prevention and Control. Surveillance of invasive bacterial diseases in Europe 2008/2009. Surveillance reports [on line] [consulted on November 2012]. Available in: http://www.ecdc.europa.eu/en/publications/Publications/1107 SUR IBD 2008-09.pdf
- Comenzó vacunación preventiva contra la meningitis en Peñalolén. Terra Noticias, 22 de octubre de 2012 [on line] [consulted on November 2012]. Available in: http://goo.gl/XUsnC0
- World Health Organization. Meningococcal vaccines: WHO position paper. November 2011.
- Servicio de Vigilancia Epidemiológica. Centro Nacional de Epidemiología. Instituto Carlos III. Comentario epidemiológico de las enfermedades de declaración obligatoria y sistema de información microbiológica. España. Año 2010. Bol Epidemiol Sem. 2011;19:100-16.
- Cano R, Garrido M. Enfermedad meningocócica en España. Análisis de la temporada 2006-2007. Bol Epidemiol Sem. 2008;16:73-84.
- Gil-Prieto R, García-García L, Álvaro-Meca A, González-Escalada A, Viguera Ester P, Gil de Miguel A. The burden of hospitalizations for meningococcal infection in Spain (1997-2008). Vaccine. 2011;29:5765-70.
- Boletín Epidemiolóxico de Galicia. A enfermdade meningocócica en Galicia: Tempada 1995/96. 1996; IX.
- Brigham KS, Sandora TJ. Neisseria meningitidis: Epidemiology, treatment and prevention in adolescents. Curr Opin Pediatr. 2009;21:437-43.
- Visintin C, Mugglestone MA, Fields EJ, Jacklin P, Murphy MS, Pollard AJ. Management of bacterial meningitis and meningococcal septicaemia in children and young people: Summary of NICE guidance. BMJ. 2010;340:c3209.
- Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: A systematic review and meta-analysis. Lancet Infect Dis. 2010;10:317-28.
- Viner RM, Booy R, Johnson H, Edmunds WJ, Hudson L, Bedford H, et al. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): A case-control study. Lancet Neurol. 2012;11:774-83.
- Orr HJ, Gray SJ, Macdonald M, Stuart JM. Saliva and meningococcal transmission. Emerg Infect Dis. 2003;9:1314-5.
- Department of Health, Victoria, Australia. Infectious diseases. Epidemiology and surveillance [on line] [consulted on February 2012]. Available in:ideas.health.vic.gov.au/bluebook/meningococcal.asp
- Caugant DA, Maiden MC. Meningococcal carriage and disease-population biology and evolution. Vaccine. 2009;27 Suppl2:B64-70.
- Fernández S, Arreaza L, Santiago I, Malvar A, Berron S, Vázquez JA, et al. Impact of meningococcal vaccination with combined serogroups A and C polysaccharide vaccine on carriage of Neisseria meningitidis C. J Med Microbiol. 2003;52:75-7.
- Claus H, Maiden MC, Wilson DJ, McCarthy ND, Jolley KA, Urwin R, et al. Genetic analysis of meningococci carried by children and young adults. J Infect Dis. 2005;191:1263-71.
- Cartwright KA, Stuart JM, Jones DM, Noah ND. The Stonehouse survey: Nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol Infect. 1987;99:591-601.
- Caugant DA, Hoiby EA, Magnus P, Scheel O, Hoel T, BjuneG, et al. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J Clin Microbiol. 1994;32:323-30.
- MacLennan J, Kafatos G, Neal K, Andrews N, Cameron JC, Roberts R, et al. Social behavior and meningococcal carriage in British teenagers. Emerg Infect Dis. 2006;12:950-7.
- Guzman-Cottrill J, Nadel S, Goldstein B. The systemic inflammatory response syndrome (SIRS), sepsis, and septic shock. In: Long SS, Pickering LK, Prober CG (eds.). Principles and practice of pediatric infectious diseases. 3rd ed. Philadelphia: Churchill Livingstone; 2008. p. 99-110.
- Pollard A, Finn A. Neisseria meningitidis. In: Long SS, Pickering LK, Prober CG (eds.). Principles and practice of pediatric infectious diseases. 3rd ed. Philadelphia: Churchill Livingstone Elsevier; 2008. p. 99-110.
- Van den Bruel A, Haj-Hassan T, Thompson M, Buntinx F, Mant D. Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: a systematic review. Lancet. 2010;375:834-45.
- Van Deuren M, Brandtzaeg P, van der Meer JW. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev. 2000;13:144-66.
- World Health Organization. Meningococcal meningitis factsheet. December 2010 [on line] [consulted on 23/10/2010]. Available in: http://www.who.int/mediacentre/factsheets/fs141/en/index.html
- American Academy of Paediatrics. Meningococo, infecciones. In: Pickering LK, Baker CJ, Kimberlyn DW, Long SS (dirs.). Red Book: Enfermedades Infecciosas en Pediatria. 28th ed. México: Editorial Médica Panamericana; 2011. p. 479-87.
- Bai X, Findlow J, Borrow R. Recombinant protein meningococcal serogroup B vaccine combined with outer membrane vesicles. Expert Opin Biol Ther. 2011;11:969-85.
- Harrison LH, Pelton SI, Wilder-Smith A, Holst J, Safadi MA, Vazquez JA, et al. The Global Meningococcal Initiative: Recommendations for reducing the global burden of meningococcal disease. Vaccine. 2011;29:3363-71.
- Safadi MA, McIntosh ED. Epidemiology and prevention of meningococcal disease: A critical appraisal of vaccine policies. Expert Rev Vaccines. 2011;10:1717-30.
- Yogev R, Tan T. Meningococcal disease: The advances and challenges of meningococcal disease prevention. Hum Vaccin. 2011;7:828-37.
- Kriz P, Wieffer H, Holl K, Rosenlund M, Budhia S, Vyse A. Changing epidemiology of meningococcal disease in Europe from the mid-20th to the early 21st Century. Expert Rev Vaccines. 2011;10:1477-86.
- De Greeff SC, de Melker HE, Spanjaard L, Schouls LM, van Derende A. Protection from routine vaccination at the age of 14 months with meningococcal serogroup C conjugate vaccine in the Netherlands. Pediatr Infect Dis J. 2006;25:79-80.
- Larrauri A, Cano R, García M, Mateo S. Impact and effectiveness of meningococcal C conjugate vaccine following its introduction in Spain. Vaccine. 2005;23:4097-100.
- Grupo de trabajo de enfermedad meningocócica de la ponencia de programa y registro de vacunación. Situación actual de la enfermedad meningocócica en España. Modificación de la pauta de vacunación frente meningitis C. Ministerio de Sanidad y Consumo, 2005.
- Cano R, Garrido M. Centro Nacional de Epidemiología, Instituto de Salud Carlos III. Enfermedad meningocócica en España. Análisis de la temporada 2006-2007. Bol Epidemiol Sem. 2008;16:73-6.
- Abad R, Vázquez JA. Microbiology and public health: new challenges in surveillance and control of meningococcal disease. Enferm Infecc Microbiol Clin. 2012;30:53-5.
- Principi N, Esposito S. Universal protein vaccines against Neisseria meningitidis serogroup B, Streptococcus pneumoniae and influenza. Hum Vaccin. 2011;7:905-12.
- Alcalá B, Salcedo C, Arreaza L, Abad R, Enríquez R, de la Fuente L, et al. Antigenic and/or phase variation of PorA protein in non-subtypable Neisseria meningitidis strains isolated in Spain. J Med Microbiol. 2004;53:515-8.
- Su EL, Snape MD. A combination recombinant protein and outermembrane vesicle vaccine against serogroup B meningococcal disease. Expert Rev Vaccines. 2011;10:575-88.
- O’Hallahan J, McNicholas A, Galloway Y, O’Leary E, Roseveare C. Delivering a safe and effective strain-specific vaccine to control an epidemic of group B meningococcal disease. N Z Med J. 2009;122:48-59.
- Rouaud P, Perrocheau A, Taha MK, Sesboue C, Forgues AM, Parent du Chatelet I, et al. Prolonged outbreak of B meningococcal disease in the Seine-Maritime department, France, January 2003 to June 2005. Euro Surveill. 2006;11:178-81.
- De Moraes JC, Perkins BA, Camargo MC, Hidalgo NT, Barbosa HA, Sacchi CT, et al. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet. 1992;340:1074-8.
- Snape MD, Dawson T, Oster P, Evans A, John TM, Ohene-Kena B, et al. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: A randomized comparative trial. Pediatr Infect Dis J. 2010;29:e71-9.
- Vázquez JA, Marcos C, Berron S. Sero/subtyping of Neisseria meningitidis isolated from patients in Spain. Epidemiol Infect. 1994;113:267-74.
- Panatto D, Amicizia D, Lai PL, Gasparini R. Neisseria meningitidis B vaccines. Expert Rev Vaccines. 2011;10:1337-51.
- Richmond PC, Marshall HS, Nissen MD, Jiang Q, Jansen KU, Garces-Sanchez M, et al. Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: A randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012;12:597-607.
- Jiang HQ, Hoiseth SK, Harris SL, McNeil LK, Zhu D, Tan C, et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine. 2010;28:6086-93.
- Serruto D, Adu-Bobie J, Capecchi B, Rappuoli R, Pizza M, Masignani V. Biotechnology and vaccines: Application of functional genomics to Neisseria meningitidisand other bacterial pathogens. J Biotechnol. 2004;113:15-32.
- Rappuoli R. Reverse vaccinology, a genome-based approach to vaccine development. Vaccine. 2001;19:2688-91.
- Moriel DG, Scarselli M, Serino L, Mora M, Rappuoli R, Masignani V. Genome-based vaccine development: A short cut for the future. Hum Vaccin. 2008;4:184-8.
- Pizza M, Scarlato V, Masignani V, Giuliani MM, Arico B, Comanducci M, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287:1816-20.
- Seib KL, Zhao X, Rappuoli R. Developing vaccines in the era of genomics: A decade of reverse vaccinology. Clin Microbiol Infect. 2012;18 Suppl 5:109-16.
- Giuliani MM, Adu-Bobie J, Comanducci M, Arico B, Savino S, Santini L, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A. 2006;103:10834-9.
- Toneatto D, Oster P, de Boer AC, Emerson A, Santos GF, Ypma E, et al. Early clinical experience with a candidate meningococcal B recombinant vaccine (rMenB) in healthy adults. Hum Vaccin. 2011;7:781-91.
- Oster P, Lennon D, O’Hallahan J, Mulholland K, Reid S, Martin D. MeNZB: A safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine. 2005;23:2191-6.
- Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, et al. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant Meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis. 2010;51:1127-37.
- Philip J, Snape MD, Robinson H, Kelly S, Pollard AJ, John TM, et al. Bactericidal antibody persistence two years following meningococcal B vaccination at 6, 8, and 12 months in 40-month old children. In: Poster presented at the European Society for Paediatric Infectious Diseases (ESPID) annual meeting. 2012. Poster No 653 [on line]. Available in: www.epostersonline.com/espid2012/?q=node/4811
- Saroey P, Snape MD, John TM, Robinson H, Kelly S, Gossger N, et al. Persistence of bactericidal antibodies following early infant immunisation with serogroup B meningococcal vaccines and immunogenicity of pre-school booster doses-a follow-on study. In: Poster presented at the European Society for Paediatric Infectious Diseases (ESPID) annual meeting. 2012. Poster No 664.
- Gossger N, Snape MD, Yu LM, Finn A, Bona G, Esposito S, et al. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: A randomized controlled trial. JAMA. 2012;307:573-82.
- Vesikari T, Esposito S, Kimura A, Kleinschmidt A, Ypma E, Toneatto D, et al. Immunogenicity of an investigational, multicomponent, meningococcall serogroup B vaccine in healthy infants at 2, 4, and 6 months of age. In: Presented at: International Pathogenic Neisseria Conference. 2010. Poster No 180 [on line]. Available in: http://neisseria.org/ipnc/2010/IPNC_2010_abstracts.pdf
- Esposito S, Vesikari T, Kimura A, Ypma E, Toneatto D, Dull P. Tolerability of a three-dose schedule of an investigational, multicomponent, meningococcal serogroup B vaccine and routine infant vaccines in a lot consistency trial. In: Presented at International Pathogenic Neisseria Conference. 2010. Poster No 182 [on line]. Available in: http://neisseria.org/ipnc/2010/IPNC_2010_abstracts.pdf
- Prymula R, Vesikari T, Esposito T, Kohl I, Ypma E, Toneatto D, et al. Catch-up vaccination of healthy toddlers with an investigational multicomponent meningococcal serogroup b vaccine (4 cmenb)-exploration of a two-dose schedule. In: Present at 29th ESPID Meeting. 2011. Poster No 706.
- Santolaya ME, O’Ryan ML, Valenzuela MT, Prado V, Vergara R, Munoz A, et al. Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4 CMenB) vaccinein healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet. 2012;379:617-24.
- Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci U S A. 2010;107:19490-5.
- Plikaytis BD, Stella M, Boccadifuoco G, Detora LM, Agnusdei M, Santini L, et al. Interlaboratory standardization of the sandwich enzyme-linked immunosorbent assay designed for MATS, a rapid, reproducible method for estimating the strain coverage of investigational vaccines. Clin Vaccine Immunol. 2012;19:1609-17.
- Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, et al. Estimating the potential strain coverage in Europe of a multicomponent vaccine targeting serogroup b meningococci. In: Oral communication presented at: 11th meeting of The European Meningococcal Disease Society (EMGM). 2011 [on line]. Available in: http://emgm.eu/meetings/emgm2011/abstracts.pdf
- Abad R, Orlandi L, Rigat F, Boccadifuoco G, Comanducci M, Muzzi A, et al. Strain coverage of a meningococcal multicomponent (4 CMenB) vaccine in Spain. In: XVIII International Pathogenic Neisseria Conference. 2012 [on line]. Available in: http://www.conventus.de/index.php?id=ipnc2012-abstracts







