Vol. 24 - Num. 93
Originales
Evolución de la vacunación frente al meningococo del serogrupo B: ¿qué ha cambiado a lo largo de 5 años?
María Vázquez Sáncheza, Cristina Genzor Ríosb, David Molina Herranza, M.ª Violeta Fariña Jaraa, Mónica López Camposc, Carmen Puig Garcíac, Carmen Viñas Viamonted, Enrique Llamas Agúndeze
aMIR-Pediatría. Hospital Universitario Miguel Servet. Zaragoza. España.
bEIR Pediatría. Hospital Universitario Miguel Servet. Zaragoza. España.
cPediatra. CS Actur Norte. Zaragoza. España.
dEnfermera de Pediatría. CS Actur Norte. Zaragoza. España.
eInformático. Sector I. Zaragoza. España.
Correspondencia: M Vázquez. Correo electrónico: mvazquezsanc@gmail.com
Cómo citar este artículo: Vázquez Sánchez M, Genzor Ríos C, Molina Herranz D, Fariña Jara MV, López Campos M, Puig García C, et al. Evolución de la vacunación frente al meningococo del serogrupo B: ¿qué ha cambiado a lo largo de 5 años? Rev Pediatr Aten Primaria. 2022;24:39-46.
Publicado en Internet: 25-02-2022 - Número de visitas: 11110
Resumen
Introducción: la infección por meningococo del serogrupo B puede provocar enfermedad meningocócica invasiva, con un 20-30% de secuelas y hasta un 10% de mortalidad.
Material y métodos: estudio observacional, descriptivo y retrospectivo de vacunación frente al meningococo del serogrupo B en la población pediátrica del Sector I de Zaragoza desde octubre de 2015 hasta diciembre de 2019. Se estudió: edad de inicio de la vacunación, edad a la fecha de la primera dosis (≤3 meses, 4-11 meses, 12-23 meses, 2-9 años, 10-16 años), sexo, centro de salud (CS) y número de dosis recibidas.
Resultados: se vacunó a 11 776 pacientes, de los cuales un 51,6% fueron varones. Presentaron una edad media de inicio de vacunación a los 5,0 ± 4,4 años y 2,2 ± 0,6 dosis recibidas. La distribución del total de vacunados fue muy variada, con una diferencia del 17,8% entre el CS con más vacunados y el CS con menos vacunados. El 0,7% recibieron primera dosis en 2015, el 23,8% en 2016, el 38% en 2017, el 26,7% en 2018 y el 10,8% en 2019. El 12% tenía ≤3 meses al inicio de la vacunación, el 11,5% tenía 4-11 meses, el 6,7% tenía 12-23 meses, el 50,4% 2-9 años y el 19,5% 10-16 años, existiendo diferencias en relación con la fecha de primera dosis (p = 0,000). El 2017 cuenta con mayor incidencia de vacunación global (12,2%), aunque en lactantes fue superior en 2018 (42,1%) y en los grupos de 2-9 años y adolescentes en 2017: el 15,8 y el 5,4% respectivamente. La incidencia global acumulada fue 32,5%, siendo en lactantes de 133,5%.
Conclusiones: a pesar de las prometedoras cifras de incidencia acumulada, encontramos numerosas diferencias de vacunación entre grupos de edad y CS, por lo que resulta interesante la vacunación sistemática y financiada de meningococo B.
Palabras clave
● Enfermedad meningocócica invasiva ● Meningococo del serogrupo B ● VacunaciónINTRODUCCIÓN
La enfermedad meningocócica es causada por la bacteria Neisseria meningitidis, un diplococo Gram negativo aerobio, con reservorio humano. Se han descrito 12 serogrupos de este patógeno, siendo los más relevantes: A, B, C, W, X, Y. La infección por este germen puede provocar cuadros de enfermedad meningocócica invasiva (EMI) en forma de meningitis y/o sepsis, con un 20-30% de secuelas y hasta un 10% de mortalidad1-3.
En España y Europa, se estiman unas tasas de incidencia de EMI de 0,3/100 000 habitantes y 0,2-14/100 000 habitantes respectivamente, con predominio del serogrupo B. Este hecho puede deberse en cierto modo a la vacunación sistemática contra el serogrupo C. Las tasas de infección varían en función de la edad, siendo la primera causa de meningitis bacteriana en niños, principalmente en menores de 2-3 años2,4.
El primer preparado vacunal frente al serogrupo B (4CmenB, Bexsero) se autorizó el 14 de enero de 2013 tras una evaluación positiva de la European Medicines Agency. Posteriormente, la agencia reguladora de cada país se encargó de su posible inclusión en calendario vacunal. El Reino Unido fue uno de los primeros países en llevarlo a cabo en marzo de 2014, con una pauta vacunal de 2 + 12.
En España, la vacuna 4CmenB está oficialmente disponible desde el 13 de agosto de 2014 en las farmacias hospitalarias y se comercializó en octubre de 2015 en las farmacias comunitarias, como una vacuna no financiada para la población general, salvo en Castilla y León y Canarias, donde sí se encuentra financiada1,2. Actualmente, existen dos preparados vacunales frente al serogrupo B en el mercado: Bexsero a partir de los 2 meses de edad y Trumemba a partir de los 10 años de edad1.
Objetivos: 1) estudiar la cobertura de la vacunación frente al meningococo del serogrupo B desde su comercialización en España hasta diciembre de 2019, previo al inicio de la pandemia por SARS-CoV-2, a través de un estudio centrado en Zaragoza; 2) analizar las diferencias de vacunación entre los diferentes grupos de edad, y 3) estudiar las posibles diferencias de incidencia de vacunación en los distintos centros de salud (CS).
MATERIAL Y MÉTODOS
Estudio de tipo observacional, descriptivo y retrospectivo, en el que se recoge información acerca de los pacientes pediátricos (hasta 16 años) vacunados frente al meningococo del serogrupo B (MenB) en el Sector I de Zaragoza desde octubre de 2015 hasta diciembre de 2019. Los 13 centros de salud que componen el Sector I de Zaragoza han sido nombrados de la "A" a la "M" de forma sucesiva.
La muestra final engloba un total de 11 776 pacientes. En cada uno de los pacientes se recogió la información relativa a las siguientes variables: fecha de nacimiento, edad de inicio de vacunación, grupo de edad en el momento de la primera dosis (≤3 meses, 4-11 meses, 12-23 meses, 2-9 años, 10-16 años), sexo, CS y número de dosis recibidas.
Los datos se recogieron en el programa IBM SPPS Statistics 20 para Windows mediante el cual se ha realizado el análisis estadístico pertinente. Los resultados se han expresado mediante medias de tendencia central (media) y medidas de dispersión (desviación típica). El test estadístico empleado ha variado en función del tipo de variable a estudio. Se han considerado estadísticamente significativos aquellos resultados que cumplían p <0,05. El proyecto inicial de investigación de este estudio fue aprobado por el Comité Ético de Investigación Clínica de Aragón.
RESULTADOS
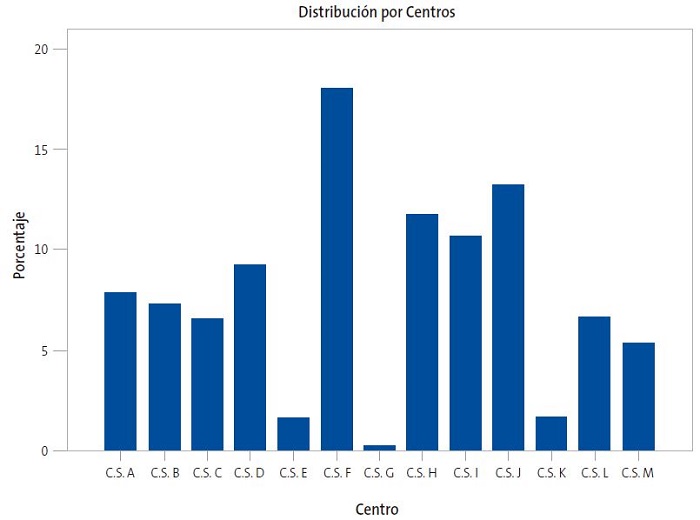
En el estudio se incluyeron 11 776 pacientes vacunados entre 2015 y 2019, con una edad media de primera dosis vacunal a los 5,0 ± 4,4 años. El 51,6% de los pacientes fueron varones y el 48,5% mujeres. Recibieron una media de 2,1 ± 0,6 dosis de la vacuna. El 18% del total de pacientes vacunados pertenecían al centro de salud "F" (CSF), seguido del CSJ, con un 13,2% de los vacunados. Los CSG, CSE y CSK son los centros con menor número de pacientes vacunados, el cual se corresponde con el 0,3%, 1,6% y 1,7% del total respectivamente. La distribución de pacientes por centro se recoge en la Fig. 1.
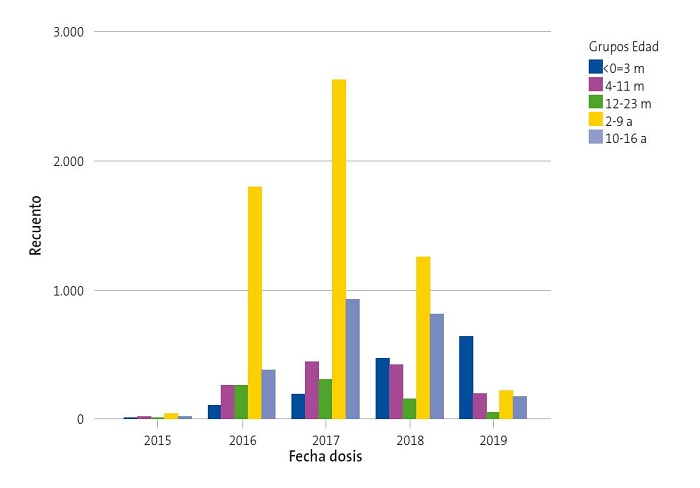
El 0,7% de los pacientes vacunados recibieron la primera dosis en 2015, el 23,8% en 2016, el 38% en 2017, el 26,7% en 2018 y el 10,8% en 2019. En cuanto a la edad de administración de primera dosis, el 12,0% tenía ≤3 meses, el 11,5% de 4 a 11 meses, el 6,7% de 12 a 23 meses, el 50,4% de 2 a 9 años y el 19,5% de 10 a 16 años. Por consiguiente, el 30,2% de los pacientes inició la vacunación en etapa de lactante. La Tabla 1 y la Fig. 2 muestran las diferencias entre grupos de edad en relación con la fecha de primera dosis de vacuna (p = 0,000). Se observa que, con el paso del tiempo, se ha incrementado el porcentaje relativo de vacunación en los menores de un año, tanto por el aumento de vacunación en este grupo como por el descenso en niños de 2-9 años y adolescentes, haciéndose estas diferencias más evidentes en 2018 y 2019.
| Tabla 1. Años de vacunación frente a meningococo de serogrupo B según grupos de edad | ||||||||
|---|---|---|---|---|---|---|---|---|
| Grupo Edad | Total | |||||||
| < 3 meses | 4-11 meses | 12-23 meses | 2-9 años | 10-16 años | ||||
| Fecha de la dosis | 2015 | Recuento | 6 | 13 | 9 | 42 | 12 | 82 |
| % dentro de fecha | 7,3% | 15,9% | 11,0% | 51,2% | 14,6% | 100,0% | ||
| % dentro de edad | 0,4% | 1,0% | 1,1% | 0,7% | 0,5% | 0,7% | ||
| 2016 | Recuento | 101 | 266 | 270 | 1792 | 378 | 2807 | |
| % dentro de fecha | 3,6% | 9,5% | 9,6% | 63,8% | 13,5% | 100,0% | ||
| % dentro de edad | 7,2% | 19,7% | 34,2% | 30,2% | 16,5% | 23,8% | ||
| 2017 | Recuento | 185 | 443 | 306 | 2625 | 921 | 4480 | |
| % dentro de fecha | 4,1% | 9,9% | 6,8% | 58,6% | 20,6% | 100,0% | ||
| % dentro de Edad | 13,1% | 32,8% | 38,7% | 44,3% | 40,1% | 38,0% | ||
| 2018 | Recuento | 474 | 439 | 154 | 1258 | 815 | 3140 | |
| % dentro de fecha | 15,1% | 14,0% | 4,9% | 40,1% | 26,0% | 100,0% | ||
| % dentro de edad | 33,6% | 32,5% | 19,5% | 21,2% | 35,5% | 26,7% | ||
| 2019 | Recuento | 646 | 188 | 51 | 214 | 168 | 1267 | |
| % dentro de fecha | 50,9% | 14,8% | 4,1% | 17,0% | 13,2% | 100,0% | ||
| % dentro de edad | 45,8% | 13,9% | 6,5% | 3,6% | 7,3% | 10,7% | ||
| Total | Recuento | 1412 | 1349 | 790 | 5931 | 2294 | 11776 | |
| % dentro de fecha | 12,0% | 11,5% | 6,7% | 50,4% | 19,5% | 100,0% | ||
| % dentro de edad | 100,0% | 100,0% | 100,0% | 100,0% | 100,0% | 100,0% | ||

La Tabla 2 muestra la evolución de la incidencia de vacunación por grupos de edad a lo largo de estos cinco años. La incidencia de vacunación en lactantes (<24 meses) fue superior en 2018 (42,1%), seguida de 2019 (36,7%), 2017 (33%), 2016 (20,9%) y 2015 (0,9%). El grupo de edad de 2-9 años, presenta una mayor incidencia en el inicio de la vacunación en 2017 (15,8%), seguido de 2016 (10,3%), 2018 (8,1%), 2019 (1,5%) y 2015 (0,2%). Por último, la incidencia en adolescentes de 10 a 16 también es mayor en 2017 (5,4%), frente a 2018 (4,7%), 2016 (2,2%), 2019 (1,0%) y 2015 (0,1%). El año 2017 fue en el que encontramos una mayor incidencia de vacunación global (12,2% del total). La incidencia acumulada en el Sector a lo largo de estos cinco años fue de 32,5%, siendo más alta en el grupo de lactantes (133,5%) respecto al grupo de 2-9 años (35,9%) y adolescentes (13,3%).
| Tabla 2. Incidencia de vacunación frente a meningococo del serogrupo B por grupos de edad entre 2015 y 2019 | |||||
|---|---|---|---|---|---|
| <24 meses | 2-9 años | 10-16 años | Total | ||
| 2015 | N | 3233 | 18 323 | 16 352 | 37 938 |
| Vacunados (%) | 28 (0,9) | 42 (0,2) | 12 (0,1) | 82 (0,2) | |
| 2016 | N | 3054 | 17 402 | 16 702 | 37 158 |
| Vacunados (%) | 637 (20,9) | 1792 (10,3) | 378 (2,3) | 2807 (7,6) | |
| 2017 | N | 2830 | 16 589 | 17 183 | 36 602 |
| Vacunados (%) | 934 (33) | 2625 (15,8) | 921 (5,4) | 4480 (12,2) | |
| 2018 | N | 2536 | 15 578 | 17 518 | 36 632 |
| Vacunados (%) | 1067 (42,1) | 1258 (8,1) | 815 (4,7) | 3140 (8,8) | |
| 2019 | N | 2414 | 14 697 | 17 694 | 34 805 |
| Vacunados (%) | 885 (36,7) | 214 (1,5) | 168 (1,0) | 1267 (3,6) | |

Se evidenciaron diferencias significativas en la edad media de primera dosis entre los distintos centros (rango de edad media entre 3,5 ± 3,9 y 6,1 ± 4,9 años; p = 0,000). Esta edad media de primera dosis también varió según la fecha de vacunación (5,6 ± 4,2 en 2017 frente a 2,6 ± 4,5 años en 2019; p = 0,000).
La distribución de la variable sexo fue homogénea en relación con la edad media (p = 0,8), CS (p = 0,9), fecha de primera dosis (p = 0,9), número de dosis recibidas (p = 0,8) y grupo de edad (p = 0,6).
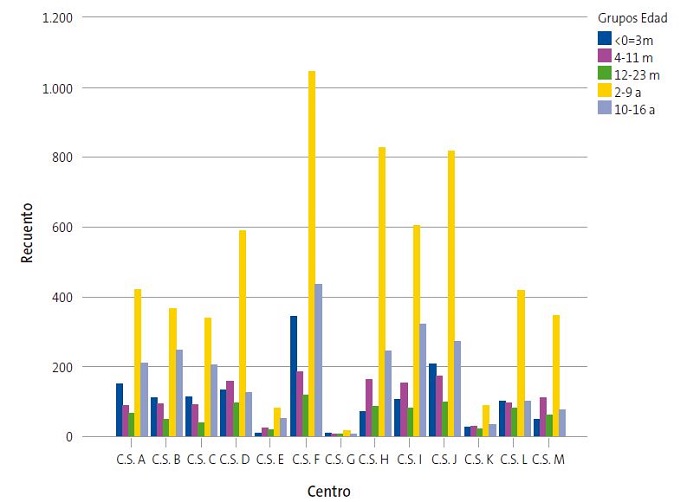
Se hallaron diferencias significativas (p = 0,000) en la asociación de CS y grupo de edad (Fig. 3). El 24,3% de pacientes que iniciaron su vacunación con edad ≤3 meses, pertenece al CSF (N = 343) y el 19% de niños que recibieron la primera dosis vacunal en etapa adolescente (10-16 años) también pertenecía a este centro. El CSG cuenta con una de las poblaciones pediátricas más reducidas y destaca un mayor porcentaje relativo de vacunación en el grupo de lactantes de <24 meses frente a otros centros, ya que los lactantes suponen el 46,6% del total de vacunados en el CSG. Asimismo, otros centros de población pediátrica reducida como el CSK, CSL y CSM, representan también cifras elevadas de vacunación en lactantes (36,6, 34,7 y 33,4% respectivamente) con respecto a otras edades. En contraposición, el CSB es uno de los centros con mayor porcentaje relativo de adolescentes vacunados (28,5%) en comparación a otros grupos de edad.
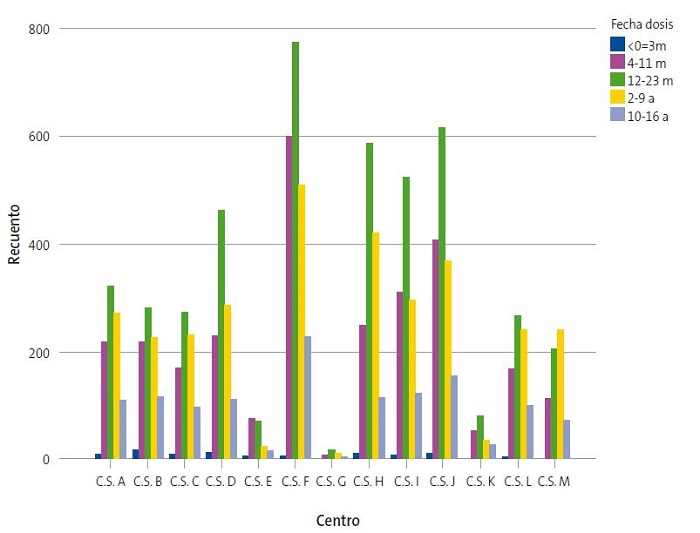
Por último, también se observaron diferencias entre CS en la incidencia de vacunación por fecha (p = 0,000). El 2017 representa el año con mayores tasas vacunales en la mayoría de los centros (Fig. 4). Entre las diferencias más notables destaca que, en el CSE, el mayor número de vacunaciones (41,2%) se iniciaron en 2016 y posteriormente fueron en descenso, mientras que en el CSM la cifra de vacunaciones fue incrementándose hasta 2019, año que recoge el inicio del 38,2% de su total de vacunaciones.
| Figura 4. Distribución de pacientes pediátricos vacunados en los 13 CS que componen el Sector I de Zaragoza entre 2015-2019 |
|---|
 |

DISCUSIÓN
El estudio realizado cuenta con una muestra significativa de la población de Zaragoza: 11 776 pacientes pediátricos. En los resultados obtenidos, destaca la diferencia de vacunación entre los distintos centros a lo largo de estos años. La diferencia de incidencia entre centros podría deberse a múltiples factores: contexto socioeconómico de la población adscrita al centro, medio de vida urbano o rural e incluso el grado de motivación de los propios profesionales sanitarios.
La incidencia acumulada objetivada en el estudio es alta (32,5%), aunque se encuentran grandes diferencias entre los distintos grupos de edad. Entre el grupo de lactantes y el de adolescentes encontramos una diferencia en incidencia acumulada del 120,1%, a favor de los lactantes. Asimismo, el curso evolutivo de la vacunación en ambos grupos es totalmente opuesto: mientras en lactantes la incidencia tiende al ascenso, en adolescentes se demuestra una disminución de la incidencia anual, más reseñable en 2019. Algunas causas que podrían justificar estas diferencias entre grupos de edades son la mayor facilidad de captación activa en lactantes debido a un mayor índice de frecuentación al centro de salud y la mayor incidencia de EMI en los menores de 2-3 años frente a los adolescentes. Sin embargo, los adolescentes representan un colectivo muy notable de portadores asintomáticos del patógeno. Se estima una colonización nasofaríngea de hasta el 50% en este grupo etario, por lo que su transmisión es mayor. En lo que respecta al meningococo C, se ha demostrado una reducción de la tasa de portadores nasofaríngeos con la vacunación en edad adolescente, lo cual se está estudiando también para el caso de la vacunación de menB3.
A pesar de que al inicio de la vacunación se intentó la captación activa en todas las franjas de edad pediátrica, parece haber disminuido considerablemente en los niños más mayores. Nuestra labor como pediatras es fomentar una adecuada comunicación con las familias de todos los grupos de edad, con el objetivo de mejorar la educación sanitaria y la promoción de la vacunación de MenB.
La introducción de Bexsero® en España tuvo lugar en octubre de 2015, como una vacuna no financiada para la población general. Solamente se encuentra financiada en pacientes con déficit de properdina o de los factores terminales del complemento, incluido el tratamiento con eculizumab, asplenia, esplenectomía programada o disfunción esplénica grave, anemia de células falciformes, personal de laboratorio (técnicos y microbiólogos) y pacientes con episodio previo de EMI1.
A pesar de que la evolución de la vacunación con MenB en la cohorte estudiada es satisfactoria, las tasas vacunales podrían mejorar con la financiación de la vacuna. Canarias y Castilla León son las únicas comunidades autónomas de España en las que la vacunación de MenB está financiada en los lactantes (Andalucía incorpora esta medida a final de 2021). A pesar de ello, el Comité Asesor de Vacunas de la Asociación Española de Pediatría recomienda su inclusión sistemática en todo el territorio nacional. Por su parte, algunos países europeos como Andorra, Italia, Irlanda, Lituania, Portugal, Reino Unido y San Marino ya la han incluido en su calendario vacunal financiado1.
El Reino Unido introdujo la vacunación sistemática de MenB en 2014 y se ha demostrado una reducción de enfermedad del 75% tras tres años de vacunación (intervalo de confianza del 95% [IC 95: 64 a 81])5. En una región de Quebec (Saguenay-Lac-Saint-Jean) se inició la vacunación del 83% de la población menor de 20 años en 2014, tras lo cual se ha evidenciado una reducción de enfermedad del 86% (IC 95: 2 a 98)6. En Italia se halla una efectividad vacunal del 93,6% en la Toscana (IC 95: 55,4 a 99,1) y 91% en Véneto (IC 95: 59,9 a 97,9)7. Por otra parte, Australia promueve la vacunación en adolescentes y arroja resultados prometedores en una cohorte de pacientes entre 16-19 años con un impacto vacunal del 71% (IC 95: 15 a 90), sin detectar nuevos casos de enfermedad entre los individuos vacunados8. En el caso de España se ha observado una disminución de incidencia de EMI por MenB en los últimos cinco años, aunque el serogrupo B continúa siendo el más prevalente en la temporada 2019-2020. Se han detectado 91 nuevos casos de MenB, que suponen el 35% de todas las EMI confirmadas: el 81,2% de los casos de EMI en menores de un año, 71,4% en el grupo de 1-4 años y 55,5% en edad 15-19 años9. Dado que en otras cohortes internacionales se ha evidenciado gran efectividad y reducción significativa de EMI en la población vacunada, resulta interesante la inclusión de la vacuna de MenB en el calendario vacunal financiado en nuestro país.
El presente estudio cuenta con varias limitaciones que podrían resultar de interés para próximas líneas de investigación. No se conoce con exactitud cómo ha afectado la pandemia por SARS-CoV-2 a la vacunación de MenB a nivel general y en los diferentes rangos de edad. Por otro lado, sería interesante conocer las características socioeconómicas de cada centro e investigar la influencia que pueden tener factores como el medio de vida (urbano/rural), la renta media por centro o el índice medio de frecuentación.
CONCLUSIONES
Existen numerosas diferencias de vacunación frente a meningococo del serogrupo B entre los distintos centros de salud, que puede deberse a las diferencias socioeconómicas y culturales de cada centro, así como de la captación activa realizada por los profesionales sanitarios.
La cobertura de vacunación en lactantes ha ido aumentando considerablemente, en contraposición al grupo de adolescentes.
CONFLICTO DE INTERESES
Los autores declaran no presentar conflictos de intereses en relación con la preparación y publicación de este artículo.
ABREVIATURAS
CS: centro de salud · EMI: enfermedad meningocócica invasiva · IC 95: intervalo de confianza del 95% · MenB: meningococo del serogrupo B.
BIBLIOGRAFÍA
- Vacuna meningococo B. En: CAV-AEP [en línea] [consultado el 22/02/2022]. Disponible en https://vacunasaep.org/familias/vacunas-una-a-una/vacuna-meningococo-b
- Moreno Pérez D, Álvarez García FJ, Aristegui Fernández J, Cilleruelo Ortega MJ, Corretger Rauet JM, García Sánchez N, et al. Vacunación frente al meningococo B. Posicionamiento del Comité Asesor de Vacunas de la Asociación Española de Pediatría. An Pediatr (Barc). 2015;82:198.e1-198.e9.
- Morillo Gutiérrez B, Berghezan Suárez A, Grupo de Patología Infecciosa de la AEPap. Vacunas antimeningocócicas en el adolescente: ¿por qué son importantes? Form Act Pediatr Aten Prim. 2018;11:145-52.
- Información Epidemiológica. Enfermedad Meningocócica - Epidemiología y situación mundial. En: Asociación de Médicos de Sanidad Exterior [en línea] [consultado el 22/02/2022]. Disponible en www.amse.es/informacion-epidemiologica/216-enfermedad-meningococica-epidemiologia-y-situacion-mundial
- Ladhani SN, Andrews N, Parikh SR, Campbell H, White J, Edelstein M, et al. Vaccination of Infants with Meningococcal Group B Vaccine (4CMenB) in England. N Engl J Med. 2020;382:309-17.
- Deceuninck G, Lefebvre B, Tsang R, Betala-Belinga JF, De Serres G, De Wals P. Impact of a mass vaccination campaign against Serogroup B meningococcal disease in the Saguenay-Lac-Saint-Jean region of Quebec four years after its launch. Vaccine. 2019;37:4243-5.
- Azzari C, Moriondo M, NiedduF, Guarnieri V, Lodi l, Canessa C, et al. Effectiveness and Impact of the 4CMenB Vaccine against Group B Meningococcal Disease in Two Italian Regions Using Different Vaccination Schedules: A Five-Year Retrospective Observational Study (2014-2018). Vaccines (Basel). 2020;8:469.
- McMillan M, Walters l, Sullivan T, Leong LEX, Turra M, Lawrence A, et al. Impact of meningococcal B (4CMenB) vaccine on pharyngeal Neisseria meningitidis carriage density and persistence in adolescents. Clin Infect Dis. 2021;73:e99-e106.
- Enfermedad meningocócica en España, temporada 2019-2020. En: CAV-AEP [en línea] [consultado el 22/02/2022]. Disponible en https://vacunasaep.org/profesionales/noticias/enfermedad-meningococica-Espana-2019-2020