Vol. 21 - Num. 82
Originales
Infección por adenovirus que requiere ingreso hospitalario: epidemiología, datos analíticos y manejo
Pedro Viaño Nogueiraa, Ana Moral Larraza, Irene Tomé Masaa, Marciano Sánchez Bayleb, Enrique Villalobos Pintoa, Marta Bascuas Arribasa, Francesco Ecclesiaa
aSección de Pediatría Hospitalaria. Hospital Infantil Universitario Niño Jesús. Madrid. España.
bPediatra. Fundación para la Investigación, Estudio y Desarrollo de la Salud Pública. Madrid. España.
Correspondencia: P Viaño . Correo electrónico: pedro.viano@salud.madrid.org
Cómo citar este artículo: Viaño Nogueira P, Moral Larraz A, Tomé Masa I, Sánchez Bayle M, Villalobos Pinto E, Bascuas Arribas M, et al. Infección por adenovirus que requiere ingreso hospitalario: epidemiología, datos analíticos y manejo. Rev Pediatr Aten Primaria. 2019;21:149-57.
Publicado en Internet: 13-06-2019 - Número de visitas: 51638
Resumen
Introducción: las infecciones por adenovirus tienen una presentación clínica variable y son una importante causa de morbilidad en la infancia. Frecuentemente reciben tratamiento antibiótico de forma innecesaria. Este estudio busca analizar las características de los pacientes con infección por adenovirus y ver si difieren de aquellos con infección bacteriana.
Pacientes y métodos: se estudiaron 174 pacientes ingresados en un hospital terciario desde enero de 2009 hasta agosto de 2017 a los que se les detectó adenovirus. Se analizaron las variables clínicas y analíticas y se compararon con las de una muestra de pacientes diagnosticados de infección bacteriana confirmada en el mismo centro en 2016.
Resultados: la tasa de pacientes con infección por adenovirus fue de 1,58/100 ingresos. El 64% eran varones, siendo la edad media de 17 meses. Los que solo presentaban síntomas gastrointestinales tenían una menor edad y resultados analíticos más favorables que los que solo mostraban síntomas respiratorios. Un 24,5% presentaban coinfección por otro virus, observándose en este grupo una mayor estancia hospitalaria (7,93 frente a 6,17 días, p = 0,006). Los criterios analíticos de infección bacteriana grave no mostraron diferencias significativas al comparar entre los pacientes infectados por adenovirus y los que tenían una infección bacteriana confirmada, excepto una diferencia mínima, aunque estadísticamente significativa, al comparar las cifras de proteína C reactiva.
Conclusiones: las variables analíticas y clínicas estudiadas no son suficientes para discriminar entre infección bacteriana y por adenovirus. Sería adecuado descartar infección por adenovirus sistemáticamente antes de instaurar tratamiento antibiótico.
Palabras clave
● Coinfección ● Infecciones del sistema respiratorio ● Infecciones por adenovirus humanos ● Reactantes de fase agudaINTRODUCCIÓN
La infección por adenovirus es una causa importante de fiebre en el niño. Las infecciones respiratorias del tracto superior, como faringitis o rinitis, e inferior, como neumonía, son los cuadros clínicos más frecuentes. Más raramente producen enfermedad gastrointestinal, genitourinaria, oftalmológica y neurológica.
Los adenovirus son un grupo de virus no envueltos con ADN de doble cadena. Se han descrito siete especies (A-G). Son clínicamente relevantes la B, C y E, asociadas a infección respiratoria; la D, que causa conjuntivitis, y la F, asociada a gastroenteritis1.
Más del 80% de los casos confirmados de infección por adenovirus se da en niños menores de cuatro años, probablemente debido a la inmadurez de su respuesta inmune humoral. Aunque la mayoría de los casos son autolimitados, tanto la enfermedad diseminada como la neumonía por adenovirus son potencialmente mortales, especialmente en niños inmunocomprometidos.
Los brotes epidémicos de enfermedad por adenovirus son especialmente frecuentes en invierno y primavera. El contagio puede producirse tanto por contacto interpersonal (inhalación de microgotas de saliva, contacto con secreción conjuntival o transmisión fecal-oral) como por fómites. Además, el virus puede acantonarse en el tejido linfoide o en el parénquima renal para posteriormente reactivarse, una situación especialmente frecuente en el paciente inmunocomprometido.
Las infecciones agudas del tracto respiratorio son una causa importante de morbimortalidad en la primera infancia en todo el mundo. Además, representan la primera causa de morbilidad infecciosa en la edad pediátrica en España2-6.
A pesar de que la mayoría de los casos se deben a virus, entre ellos el adenovirus, se realiza una prescripción excesiva de antibióticos, innecesarios para tratar este tipo de infecciones. Su uso no se asocia con mejoría clínica e incluso es potencialmente dañino, pues expone a un mayor riesgo de efectos adversos y aumenta la prevalencia de bacterias resistentes, lo que disminuye las probabilidades de que una infección bacteriana invasiva responda a la terapia antibiótica estándar3,7-12.
Sin embargo, en diversos estudios se observó cómo la mayoría de los médicos deciden no modificar el manejo antibiótico tras detectar un patógeno respiratorio viral en la muestra recogida, en parte debido a la corta edad de los pacientes y a la tendencia a completar el tratamiento antibiótico para disminuir el desarrollo de resistencias bacterianas. Aun así, solicitar pruebas para detectar patógenos virales puede ser útil para el manejo individual de los pacientes, disminuir el número de pruebas complementarias innecesarias y aplicar las medidas de aislamiento requeridas para reducir la transmisión nosocomial de las infecciones, con la consecuente disminución del tiempo de hospitalización y del coste económico que supone5.
Los objetivos del estudio son describir las características clínicas y analíticas que se asocian con la infección por adenovirus, comparar estas variables con pacientes que presentan coinfección por más de un patógeno viral y analizar las diferencias de estas entre los enfermos con adenovirus y otros con infección bacteriana comprobada.
PACIENTES Y MÉTODOS
Criterios de inclusión
Se estudiaron los pacientes ingresados en un hospital terciario en los que se detectó infección por adenovirus. El periodo estudiado abarca de enero de 2009 a agosto de 2017 (en total, ocho años y ocho meses). Se solicitó la detección a todos los pacientes ingresados con clínica sugestiva de infección respiratoria o gastrointestinal no filiada al ingreso.
Asimismo, se analizaron los pacientes diagnosticados de infección bacteriana comprobada en 2016, que fueron utilizados como controles para comparar las características analíticas relacionadas con infección.
Métodos de detección
Para la detección de adenovirus en vía respiratoria, se recogió moco nasofaríngeo mediante aspirado en Urgencias o durante el primer día de ingreso hospitalario. Posteriormente, se llevó a cabo un test comercial (Allplex Respiratory RP3, Seegene, Taewon Bldg., 91, Ogeum-ro, Songpa-gu, Seúl, Corea) de detección mediante reacción en cadena de la polimerasa a tiempo real cualitativa de 19 virus respiratorios: adenovirus, bocavirus, coronavirus NL63, 229E y OC43, enterovirus, influenza A –específicamente, también A-H1, A-H1pdm09 y A-H3– y B, metapneumovirus, parainfluenza 1 a 4, respiratorio sincitial A y B y rinovirus. Para la detección de adenovirus en vía digestiva, se recogieron heces expulsadas de forma espontánea. Posteriormente, se llevó a cabo un test comercial de detección mediante reacción en cadena de la polimerasa a tiempo real cualitativa de adenovirus.
Recogida de datos
Se recogió información sobre las siguientes variables: edad, sexo, fecha de ingreso, días totales de ingreso, días de evolución del cuadro clínico previo al ingreso, número de hermanos, presencia de convivientes fumadores, ambiente epidémico familiar previo al ingreso, fiebre al ingreso, presencia de síntomas respiratorios al ingreso, presencia de vómitos o diarrea al ingreso, saturación de oxígeno basal al ingreso, estado general (bueno, moderado, malo), concentración de leucocitos, neutrófilos, cayados y plaquetas, concentración de proteína C reactiva y procalcitonina, infección por otros virus respiratorios, calificación de la radiografía de tórax por el radiólogo (normal, anormal), antibioterapia recibida durante el ingreso y oxigenoterapia recibida durante el ingreso.
Análisis de datos
Se realizaron todos los análisis estadísticos mediante el programa comercial IBM SPSS Statistics, versión 15.0. Se fijó un nivel de significación del 0,05. Los resultados se expresaron mediante intervalos de confianza del 95%.
Para la comparación de variables cualitativas se empleó el test χ2 de Pearson y el test exacto de Fisher. Se empleó la prueba de Kolmogorov-Smirmov para determinar si las variables cuantitativas seguían una distribución normal. Si la seguían se utilizó el test t de Student. En caso contrario se empleó la prueba U de Mann-Whitney.
En el análisis de pruebas diagnósticas se calcularon las odds ratio y el área bajo la curva de las curvas ROC.
RESULTADOS
Durante el periodo estudiado se produjeron un total de 174 ingresos con infección por adenovirus, de un total de 10 990 hospitalizaciones en la sección de Pediatría general del centro, lo que supone una tasa global de pacientes con infección por adenovirus de 1,58/100 ingresos, con una alta variabilidad interanual (rango de 0,41-2,18). Respecto a la distribución por meses, más de la mitad de los casos se repartieron entre los meses de marzo (17,2%), abril (12,6%), mayo (10,9%) y diciembre (10,9%), mientras que agosto es el mes con menos ingresos por esta causa (1,7%). Del total de la muestra, el 64,4% de los pacientes eran varones, siendo la edad media de 17 meses. Los parámetros analíticos muestran una gran variabilidad, dado que se observa un número considerable de pacientes con parámetros indicativos de infección bacteriana grave (leucocitos >15 000/µl, proteína C reactiva [PCR] >3 mg/dl, procalcitonina [PCT] >1 ng/ml), incluso considerando que en un 24% de los pacientes no se realizó analítica. Las características generales de todos los pacientes se recogen en la Tabla 1.
| Tabla 1. Características de los pacientes con adenovirus | |
|---|---|
| Edad (años), media ± DE (mínimo; máximo) | 1,39 ± 1,32 (0,8; 7,8) |
| Hombres, n (%) | 112 (64,4) |
| Fiebre al ingreso, n (%) | 119 (68,4) |
| Tiempo de evolución (días), media ± DE (mínimo; máximo) | 4,16 ± 4,28 (0,1;30) |
| Leucocitos ×1000/μl, media ± DE (mínimo; máximo) | 15,1 ± 8,24 (2,32; 56,4) |
| Neutrófilos ×1000/μl, media ± DE (mínimo; máximo) | 8,93 ± 6,95 (0,39; 50,41) |
| Proteína C reactiva (mg/dl), media ± DE (mínimo; máximo) | 4,88 ± 5,32 (0,06; 29,3) |
| Procalcitonina (ng/ml) media ± DE (mínimo; máximo) | 1,65 ± 2,11 (0,1; 15) |
| Leucocitos >15 000/μl, n (%) | 61 (35,1) |
| Proteína C reactiva >3 (mg/dl), n (%) | 63 (36,2) |
| Procalcitonina >1 (ng/ml), n (%) | 23 (13,2) |

Del total de los encuestados hubo 20 con padres fumadores (11,5%) y 8 (4,6%), con ambiente epidémico.
Se recogieron 54 casos con síntomas gastrointestinales, asociándose en un 44,4% a síntomas respiratorios. En la Tabla 2 se comparan las características de aquellos que manifestaron únicamente síntomas gastrointestinales respecto a quienes exclusivamente presentaron sintomatología respiratoria. Se observa una menor edad y resultados analíticos más favorables en aquellos pacientes que solo presentaban síntomas gastrointestinales respecto a los que únicamente presentaban síntomas respiratorios.
| Tabla 2. Comparación entre infecciones gastrointestinales (GEA) o respiratorias por adenovirus | |||
|---|---|---|---|
| GEA | Respiratorio | Valor de p | |
| Días ingreso, media ± DE | 6 ± 3 | 7 ± 4 | >0,05 |
| Edad (años), media ± DE | 0,77 ± 0,59 | 1,54 ± 1,50 | 0,006 |
| Leucocitos ×1000/μl, media ± DE | 13,16 ± 8,54 | 16,01 ± 8,83 | 0,036 |
| Neutrófilos ×1000/μl, media ± DE | 6,21 ± 4,91 | 9,94 ± 8,18 | 0,005 |
| Proteína C reactiva (mg/dl), media ± DE | 2,98 ± 4,83 | 5,26 ± 5,57 | 0,01 |
| Procalcitonina (ng/ml), media ± DE | 1,01 ± 1,86 | 1,25 ± 2,47 | >0,05 |

Un total de 92 (52,9%) pacientes recibieron tratamiento antibiótico en Urgencias o en algún momento del ingreso hospitalario.
Casi un tercio de los pacientes (51) presentaban coinfección por otro virus: 11% con VRS, 8,2% con rinovirus, 5,5% con rotavirus, 2,2% con bocavirus y 1% con influenzavirus.
La Tabla 3 compara las características de los pacientes según tuvieran o no coinfección. Aquellos pacientes con coinfección presentaban una mayor estancia hospitalaria de forma estadísticamente significativa. A su vez, mostraban una tendencia a tener la temperatura corporal y los parámetros analíticos más elevados, a excepción de la variable leucocitos >15 000/μl. Por último, cabe destacar que apenas hay diferencia de edad entre ambos grupos.
| Tabla 3. Comparación entre pacientes con y sin coinfección | |||
|---|---|---|---|
| Coinfección | Sin coinfección | valor de p | |
| Días ingreso, media ± DE | 7,93 ± 4,45 | 6,17 ± 4,25 | <0,006 |
| Edad (años), media ± DE | 1,20 ± 1,32 | 1,45 ± 1,30 | >0,05 |
| Temperatura (°C) | 37,61 ± 1,05 | 37,40 ± 1,04 | >0,05 |
| Leucocitos ×1000/µl, media ± DE | 16,18 ± 10,03 | 14,51 ± 7,28 | >0,05 |
| Neutrófilos ×1000/µl, media ± DE | 9,37 ± 9,17 | 8,67 ± 5,76 | >0,05 |
| Proteína C reactiva (mg/dl), media ± DE | 5,92 ± 6,12 | 4,48 ± 5,03 | >0,05 |
| Procalcitonina (ng/ml), media ± DE | 1,27 ± 1,78 | 1,14 ± 2,31 | >0,05 |
| Leucocitos >15 000, n (%) | 16 (40) | 45 (48,9) | >0,05 |
| Proteína C reactiva >3 (mg/dl), n (%) | 21 (58,3) | 43 (47,2) | >0,05 |
| Procalcitonina >1 (ng/ml), n (%) | 10 (34,4) | 14 (24,1) | >0,05 |
| Plaquetas >400 000/µl, n (%) | 22 (51,1) | 46 (35,3) | >0,05 |
| Toma de antibióticos, n (%) | 26 (59) | 66 (48,5) | >0,05 |
| Ingreso en UCI, n (%) | 5 (11,1) | 11 (8,2) | >0,05 |
| Hemocultivo positivo, n (%) | 2 (4,6) | 1 (0,7) | >0,05 |
| Oxigenoterapia, n (%) | 23 (56,1) | 64 (50) | >0,05 |

Al comparar la población seleccionada con pacientes que presentan una infección bacteriana confirmada por cultivo vemos que los 3 criterios analíticos de infección bacteriana grave (leucocitos >15 000/μl, PCR >3 mg/dl, PCT >1 ng/ml), como se observa en la Tabla 4, no muestran diferencias significativas. Estos resultados concuerdan con los datos que se muestran en la curva ROC de la Fig. 1. Se observa que los parámetros analíticos son poco útiles para discriminar entre una infección bacteriana y una por adenovirus. Como puede verse en la Tabla 5, únicamente el área bajo la curva (ABC) de la PCR tiene significación estadística, aunque con mínima capacidad de discriminación entre infección bacteriana y por adenovirus.
| Tabla 4. Comparación entre pacientes con infección bacteriana y con adenovirus | ||||
|---|---|---|---|---|
| Infección bacteriana | Adenovirus | OR | IC | |
| Al menos un criterio | 92 | 62 | 1,30 | (0,66-2,77) |
| Al menos dos criterios | 58 | 33 | 1,54 | (0,84-2,84) |
| Los tres criterios | 23 | 12 | 1,48 | (0,65-3,41) |

| Figura 1. Curva ROC de los parámetros analíticos para la comparación entre infección bacteriana y por adenovirus |
|---|
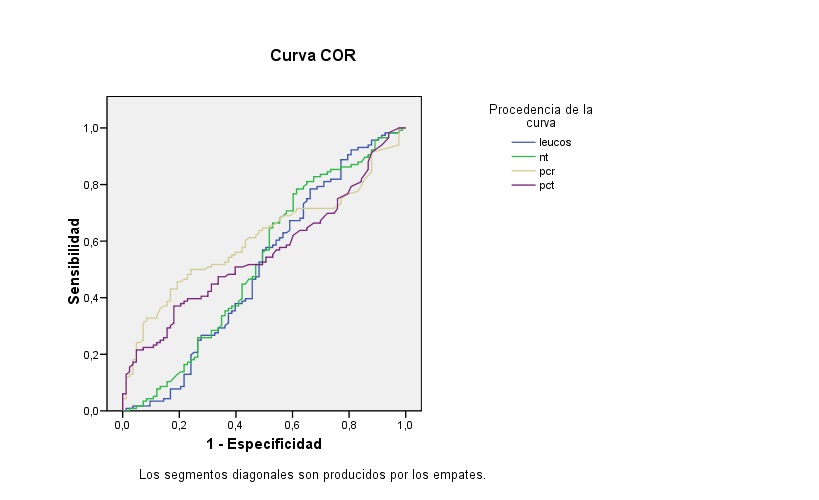 |

| Tabla 5. Área bajo la curva de los parámetros analíticos para la comparación entre infección bacteriana y por adenovirus | ||||
|---|---|---|---|---|
| Variables resultado de contraste | Área | p | Intervalo de confianza del 95% | |
| Límite superior | Límite superior | |||
| Leucocitos | 0,508 | 0,850 | 0,423 | 0,593 |
| Neutrófilos totales | 0,527 | 0,514 | 0,442 | 0,612 |
| Proteína C reactiva | 0,609 | 0,009 | 0,531 | 0,687 |
| Procalcitonina | 0,559 | 0,159 | 0,479 | 0,638 |

DISCUSIÓN
La frecuencia de infección por adenovirus en el estudio ha sido de 1,58/100 ingresados en la sección de Pediatría General del centro, con una gran variabilidad anual, que probablemente se debe a un sesgo de detección.
La época del año en la que ingresaron más pacientes diagnosticados de infección por adenovirus fue en primavera. El 64,4% de los casos recogidos eran varones. Ambas características coinciden con los estudios descritos en la literatura científica5.
Un 24,5% de los pacientes presentaba coinfección por otro virus respiratorio, un porcentaje similar al observado por otros autores5,13. Dentro del grupo de pacientes con coinfección, un 11% se asociaba a virus respiratorio sincitial (VRS) y un 8,2%, a rinovirus. Estos dos virus son los que se detectaron con más frecuencia en muestras respiratorias de pacientes con infección aguda del tracto respiratorio en la mayoría de los estudios analizados, lo que podría explicar que hayan sido los agentes infecciosos más frecuentemente asociados a adenovirus en el estudio5.
Sobre la edad de los pacientes, se encontraron diferencias significativas entre los casos en función de la coinfección, ya que la coinfección es más frecuente cuanto menor es el paciente. Estos datos coinciden con los de otros autores que refieren que la tasa de coinfección es menor en niños mayores5,14, lo que podría atribuirse a que los más pequeños presentan un menor aclaramiento viral, pues su inmunidad frente a virus es más inmadura5.
Respecto a la necesidad de oxigenoterapia suplementaria, en el estudio no se hallaron diferencias significativas entre los grupos de pacientes con coinfección por virus respiratorios y los que no la tenían, algo similar a lo descrito en la mayoría de los estudios revisados, aunque en algunos la coinfección se asoció con menor requerimiento de oxigenoterapia5,13.
Además, los pacientes con coinfección por virus respiratorios presentaron una mayor estancia hospitalaria que los infectados únicamente por adenovirus. Este hallazgo difiere con los resultados obtenidos en otros estudios, en los que los pacientes con un solo patógeno respiratorio tenían una media de hospitalización mayor en comparación con pacientes con coinfección por virus respiratorios5.
Se han señalado resultados contradictorios sobre la asociación entre coinfección y gravedad de la enfermedad aguda. Algunos señalan que la clínica es más grave si la infección estaba provocada por múltiples patógenos virales15-21, mientras que otros no encontraron diferencias significativas en la gravedad entre los casos producidos con y sin coinfección viral18,22-31. Incluso en algunas publicaciones los pacientes con coinfección viral ingresaban con menos frecuencia en unidades de cuidados intensivos que los infectados por un solo virus, lo que podría deberse a coinfecciones virus-bacteria no detectadas, aunque evidentemente convendría analizar los diferentes tipos de coinfecciones encontradas5,13,30,32-37.
Respecto a las variables analíticas, la PCR es un reactante de fase aguda que indica lesión tisular, ya sea debido a infección o a procesos como traumatismo o inflamación, por lo que es esencial tener en cuenta el contexto clínico del paciente a la hora de interpretar el resultado y es importante recordar que no permite diferenciar con certeza entre infección de etiología vírica y bacteriana3, aunque en algunos estudios se observó que una PCR sérica superior a 40-60 mg/l era significativamente más frecuente en infecciones bacterianas38.
En el estudio los niveles de PCR sérica mayores de 3 mg/dl fueron más frecuentes en el grupo con coinfección (58,3%) que en el grupo infectado solo por adenovirus (47,2%). Asimismo, la media del nivel de PCR sérica era mayor en el primer grupo. Pero en ambos casos las diferencias no son estadísticamente significativas.
Si comparamos algunos datos analíticos sugestivos de infección bacteriana grave (leucocitos >15 000/μl, PCR >3 mg/dl, PCT >1 ng/ml) de los pacientes incluidos en la muestra con los de pacientes con infección bacteriana confirmada, los resultados no muestran diferencias significativas, lo que constituye un ejemplo de la limitada utilidad de las variables analíticas para clasificar la etiología de una infección como vírica o bacteriana, lo que también se evidencia en el ABC encontrado para los parámetros analíticos estudiados que no supera 0,609 (caso de la PCR, el mejor valor encontrado). Se ha señalado que las infecciones virales respiratorias aumentan la susceptibilidad a infecciones bacterianas, al alterar las barreras físicas y el sistema inmune39.
Las principales limitaciones del estudio han sido las intrínsecas a los estudios retrospectivos y que, al tratarse exclusivamente de pacientes ingresados, se pierde información sobre los casos de infecciones manejadas de manera ambulatoria. Por otro lado, la PCR puede tener valores ligeramente elevados una vez pasada la infección5,14,40, si bien los análisis bacteriológicos fueron negativos en todos ellos.
En conclusión, la infección por adenovirus es una causa frecuente de hospitalización pediátrica, especialmente en varones y en primavera. Frecuentemente se asocia a infecciones por otros virus, lo que aumenta la estancia hospitalaria y los valores de PCR. Los parámetros analíticos habituales indicativos de infección no permiten distinguir etiología vírica de bacteriana por lo que sería conveniente descartar infección por adenovirus sistemáticamente antes de instaurar tratamiento antibiótico. Son necesarios más estudios que definan con mayor detalle las características y asociaciones del paciente infectado por adenovirus.
CONFLICTO DE INTERESES
Los autores declaran no presentar conflictos de intereses en relación con la preparación y publicación de este artículo.
ABREVIATURAS
PCR: proteína C reactiva · PCT: procalcitonina · VRS: virus respiratorio sincitial.
BIBLIOGRAFÍA
- Lynch JP, Kajon AE. Adenovirus: epidemiology, global spread of novel serotypes, and advances in treatment and prevention. Semin Respir Crit Care Med. 2016;37:586-602.
- Marcone DN, Durand LO, Azziz-Baumgartner E, Vidaurreta S, Ekstrom J, Carballal G, et al. Incidence of viral respiratory infections in a prospective cohort of outpatient and hospitalized children aged ≤5 years and its associated cost in Buenos Aires, Argentina. BMC Infect Dis. 2015;15:447.
- Llor C, Alkorta M, de la Flor J, Bernárdez S, Cañada JL, Bárcena M, et al. Recomendaciones de utilización de técnicas de diagnóstico rápido en infecciones respiratorias en Atención Primaria. Aten Primaria. 2017;49:426-37.
- Jevsnik M, Ursic T, Zigon N, Lusa L, Krivec U, Petrovec M. Coronavirus infections in hospitalized pediatric patients with acute respiratory tract disease. BMJ Infect Dis. 2012;12:365.
- Huijskens EG, Biesmans RC, Buiting AG, Obihara CC, Rossen JW. Diagnostic value of respiratory virus detection in symptomatic children using real-time PCR. Virol J. 2012;9:276.
- Kahn JS. Newly discovered respiratory viruses: significance and implications. Curr Opin Pharmacol. 2007;7:478-83.
- Rosenstein N, Phillips WR, Gerber MA, Marcy SM, Schwartz B, Dowell SF. The common cold-Principles of judicious use of antimicrobial agents. Pediatrics. 1998;101:181-4.
- Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735-43.
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patientes: systematic review and meta-analysis. BMJ. 2010;340:c2096.
- Dowell SF, Schwartz B. Resistant pneumococci: protecting patients through judicious antibiotic use. Am Fam Physician. 1997;55:1647-54.
- Van de Pol AC, Wolfs TF, Jansen NJ, van Loon AM, Rossen JW. Diagnostic value of real-time polymerase chain reaction to detect viruses in young children admitted to the paediatric intensive care unit with lower respiratory tract infection. Crit Care. 2006;10:R61.
- Wishaupt JO, Russcher A, Smeets LC, Versteegh FG, Hartwig NG. Clinical impact of RT-PCR for pediatric acute respiratory infections: a controlled clinical trial. Pediatrics. 2011;128:e1113-20.
- Chorazy ML, Lebeck MG, McCarthy TA, Richter SS, Torner JC, Gray GC. Polimicrobial acute respiratory infections in a hospital-based pediatric population. Pediatr Infect Dis J. 2013;32:460-6.
- Cebey-López M, Herberg J, Pardo-Seco J, Gómez-Carballa A, Martinón-Torres N, Salas A, et al. Viral co-infections in pediatric patients hospitalized with lower tract acute respiratory infections. PLoS One. 2015;10:e0136526.
- Bharaj P, Sullender WM, Kabra SK, Mani K, Cherian J, Tyagi V, et al. Respiratory viral infections detected by multiplex PCR among pediatric patients with lower respiratory tract infections seen at an urban hospital in Delhi from 2005 to 2007. Virol J. 2009;6:89.
- Bonzel L, Tenenbaum T, Schroten H, Schildgen O, Schweitzer-Krantz S, Adams O. Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real-time polymerase chain reaction. Pediatr Infect Dis J. 2008;27:589-94.
- Jennings LC, Anderson TP, Beynon KA, Chua A, Laing RT, Werno AM, et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63:42-8.
- Midulla F, Scagnolari C, Bonci E, Pierangeli A, Antonelli G, De Angelis D, et al. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Child. 2010;95:35-41.
- Templeton KE, Scheltinga SA, van den Eeden WC, Graffelman AW, van den Broek PJ, Claas EC. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41:345-51.
- Yoshida LM, Suzuki M, Yamamoto T, Nguyen HA, Nguyen CD, Nguyen AT, et al. Viral pathogens associated with acute respiratory infections in central vietnamese children. Pediatr Infect Dis J. 2010;29:75-7.
- Paranhos-Baccalà G, Komurian-Pradel F, Richard N, Vernet G, Lina B, Floret D. Mixed respiratory virus infections. J Clin Virol. 2008;43:407-10.
- Gil J, Almeida S, Constant C, Pinto S, Barreto R, Cristino JM, et al. Relevancia a corto plazo de la coinfección viral en pacientes menores de 2 años hospitalizados con infecciones de las vías respiratorias inferiores. An Pediatr (Barc). 2018;88:127-35.
- Reina J, Ferrés F, Rubio R, Rojo-Molinero E. Análisis de las coinfecciones detectadas entre los subtipos del virus respiratorio sincitial y otros virus respiratorios. An Pediatr (Barc). 2015;82:e255-6.
- Harada Y, Kinoshita F, Yoshida LM, Minh LN, Suzuki M, Morimoto K, et al. Does respiratory virus coinfection increases the clinical severity of acute respiratory infection among children infected with respiratory syncytial virus? Pediatr Infect Dis J. 2013;32:441-5.
- Suryadevara M, Cummings E, Bonville CA, Bartholoma N, Riddell S, Kiska D, et al. Viral etiology of acute febrile respiratory illnesses in hospitalized children younger than 24 months. Clin Pediatr (Phila). 2011;50:513-7.
- Brand HK, de Groot R, Galama JM, Brouwer ML, Teuwen K, Hermans PW, et al. Infection with multiple viruses is not associated with increased disease severity in children with bronchiolitis. Pediatr Pulmonol. 2012;47:393-400.
- Peng D, Zhao D, Liu J, Wang X, Yang K, Xicheng H, et al. Multipathogen infections in hospitalized children with acute respiratory infections. Virol J. 2009;6:155.
- Brouard J, Freymuth F, Vabret A, Jokic M, Guillois B, Duhamel JF. Co-infections virales lors des bronchiolites du nourrisson immunocompetent: etude prospective epidemiologique. Arch Pediatr. 2000;7 Suppl 3:S531-5.
- Drews AL, Atmar RL, Glezen WP, Baxter BD, Piedra PA, Greenberg SB. Dual respiratory virus infections. Clin Infect Dis. 1997;25:1421-9.
- Fabbiani M, Terrosi C, Martorelli B, Valentini M, Bernini L, Cellesi C, et al. Epidemiological and clinical study of viral respiratory tract infections in children from Italy. J Med Virol. 2009;81:750-6.
- Jennings LC, Anderson TP, Beynon KA, Chua A, Laing RT, Werno AM, et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63:42-8.
- Van der Zalm MM, van Ewijk BE, Wilbrink B, Uiterwaal CS, Wolfs TF, van der Ent CK. Respiratory pathogens in children with and without respiratory symptoms. J Pediatr. 2009;154:396-400, 400 e1.
- Aberle JH, Aberle SW, Pracher E, Hutter HP, Kundi M, Popow-Kraupp T. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon-gamma response. Pediatr Infect Dis J. 2005;24:605-10.
- Choi EH, Lee HJ, Kim SJ, Eun BW, Kim NH, Lee JA, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005. Clin Infect Dis. 2006;43:585-92.
- Huang JJ, Huang TY, Huang MY, Chen BH, Lin KH, Jeng JE, et al. Simultaneous multiple viral infections in childhood acute lower respiratory tract infections in southern Taiwan. J Trop Pediatr. 1998;44:308-11.
- Legg JP, Warner JA, Johnston SL, Warner JO. Frequency of detection of picornaviruses and seven other respiratory pathogens in infants. Pediatr Infect Dis J. 2005;24:611-6.
- Peng D, Zhao D, Liu J, Wang X, Yang K, Xicheng H, et al. Multipathogen infections in hospitalized children with acute respiratory infections. Virol J. 2009;6:155.
- Flood RG, Badik J, Aronoff SC. The utility of serum C-reactive protein in differentiating bacterial from nonbacterial pneumonia in children: a meta-analysis of 1230 children. Pediatr Infect Dis J. 2008;27:95-9.
- Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23:74-98.
- Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72:695-9.





