Vol. 20 - Num. 77
Originales
Asociación del consumo de lácteos con las infecciones respiratorias: ¿mito o realidad?
Diego Mauricio Peñafiel Freirea, Nerea Martín Calvob, Lorena García Blancoc, Itziar Zazped, Noelia Álvarez Zalloe, Laura Moreno Galarragaf
aServicio de Pediatría. Complejo Hospitalario de Navarra. Pamplona. España.
bDepartamento de Medicina Preventiva y Salud Pública. Universidad de Navarra. Pamplona. España.
cPediatra. CS Ansoáin. Ansoáin. Navarra. España.
dDepartamento de Ciencias de la Alimentación y Fisiología. Universidad de Navarra. Pamplona. España.
eServicio de Urgencias Extrahospitalarias. Servicio Navarro de Salud-Osasunbidea. Pamplona. España.
fServicio de Pediatría. Complejo Hospitalario de Navarra. Servicio Navarro de Salud. Pamplona. Instituto de Investigación Sanitaria de Navarra (IdiSNA). Pamplona. España.
Correspondencia: DM Peñafiel. Correo electrónico: dpfreire.89@gmail.com
Cómo citar este artículo: Peñafiel Freire DM, Martín Calvo N, García Blanco L, Zazpe I, Álvarez Zallo N, Moreno Galarraga L. Asociación del consumo de lácteos con las infecciones respiratorias: ¿mito o realidad? Rev Pediatr Aten Primaria. 2018;20:45-52.
Publicado en Internet: 15-02-2018 - Número de visitas: 50933
Resumen
Introducción: la leche y los derivados lácteos son alimentos importantes para el desarrollo. Sin embargo, la creencia de que se asocian con infecciones respiratorias está provocando que se limite su consumo o se sustituya por bebidas vegetales. El objetivo del estudio fue analizar si el consumo de lácteos se asocia con determinadas infecciones respiratorias en la infancia.
Material y métodos: estudio transversal con 169 voluntarios de entre 4 y 7 años. Información recogida mediante cuestionarios en papel. Información dietética recogida mediante cuestionario de frecuencia de consumo de alimentos semicualitativo de 151 ítems. Se valoró la asociación del consumo de leches, quesos y yogures con determinadas enfermedades respiratorias (otitis media aguda, sinusitis, mastoiditis, neumonía), comparando dos categorías de consumo definidas a partir de la mediana de cada alimento, mediante regresión logística multivariable.
Resultados: no se encontraron asociaciones entre el consumo de lácteos y las enfermedades respiratorias analizadas (odds ratio: 0,85; intervalo de confianza del 95%: 0,44 a 1,64]). Al analizar cada lácteo por separado, se encontró una asociación inversa entre el consumo de quesos y las enfermedades respiratorias en conjunto (odds ratio: 0,50; intervalo de confianza del 95%: 0,26 a 0,98), pero no para cada una de las infecciones por separado (otitis media aguda ni neumonía). No se encontró asociación significativa con los desenlaces para ningún otro derivado lácteo (leche o yogures).
Conclusiones: los resultados no apoyan una asociación directa entre el consumo de leche y derivados con infecciones respiratorias en la infancia. Con los datos actuales no está justificado restringir el consumo de leche o derivados en niños en edad escolar.
Palabras clave
● Infecciones del sistema respiratorio ● Neumonía ● Otitis media ● Productos lácteosINTRODUCCIÓN
Existe una extendida creencia social que relaciona el consumo de productos lácteos con la aparición de diversas patologías respiratorias en la infancia1,2. En concreto, se cree que el consumo de proteínas de leche de vaca (PLV) se asocia con un aumento de la mucosidad a nivel respiratorio y de las infecciones respiratorias. A pesar de que la evidencia disponible no lo justifica3, en la práctica clínica es frecuente que los padres de niños con infecciones respiratorias de repetición limiten la ingesta de PLV o las sustituyan por proteínas vegetales, fórmulas sin lactosa o derivados de soja o arroz.
Los lácteos son un alimento fundamental para el desarrollo físico del lactante y del niño4, y también se han asociado con un mejor rendimiento académico5. Además, el contenido en calcio de los lácteos resulta beneficioso para el adecuado desarrollo de la masa ósea y para el control de la presión arterial6-8. En edad escolar, el consumo recomendado de lácteos es de 2-4 raciones diarias9.
Varios estudios transversales han sugerido incluso que el consumo de lácteos podría ejercer un efecto protector frente a patología respiratoria. Un estudio realizado en Nueva Zelanda encontró que el consumo de leche y huevos en los 12 meses previos se asociaba con una reducción significativa de la incidencia de enfermedades sibilantes10. En la misma línea, se ha descrito una mayor incidencia de síntomas respiratorios, principalmente los relacionados con bronquitis y asma, en personas con una menor ingesta de leche11-12. En cuanto a la producción de mucosidad, no se han encontrado diferencias entre el placebo a base de soja y la leche de vaca13.
Sin embargo, la presión social, el aumento de la oferta de alternativas a la leche y derivados, y la falta de evidencia científica están provocando que muchos padres limiten el consumo de lácteos de sus hijos, o que lo sustituyan por bebidas de soja o arroz. Por ello, el objetivo de este estudio es analizar la asociación del consumo de leche de vaca y derivados lácteos (quesos y yogures) con una serie de enfermedades respiratorias en la infancia.
MATERIAL Y MÉTODOS
Diseño del estudio
Estudio transversal que compone la fase piloto del proyecto Seguimiento de Escolares Navarros para un Desarrollo Óptimo (SENDO), una cohorte pediátrica, prospectiva y multipropósito, dirigida al estudio del impacto de la dieta y el estilo de vida sobre el desarrollo de diferentes patologías del niño y del adolescente. Se dispone de más información acerca del proyecto SENDO en su página web (www.proyectosendo.es).
Selección de la muestra
El reclutamiento de los participantes se llevó a cabo a través de los pediatras de Atención Primaria del Servicio Navarro de Salud-Osasunbidea (SNS-O) entre febrero y abril de 2015. Se trata de una muestra compuesta por voluntarios, donde los padres o representantes legales de todos los participantes firmaron un consentimiento informado para participar en el estudio. Como criterio de inclusión, los participantes debían ser residentes en la Comunidad Foral de Navarra (provincia situada al norte de España), y haber nacido entre enero de 2008 y diciembre de 2010. No se establecieron criterios de exclusión.
La muestra inicial estaba compuesta por 170 niños que, a fecha de septiembre de 2016, habían completado y remitido al equipo investigador el cuestionario basal. Un participante fue excluido por reportar un consumo energético fuera de los límites establecidos (entre 550 y 3800 kcal/día)14, por lo que la muestra final estaba compuesta por 169 menores.
Recogida de información
Información sobre la exposición
La información se recogió a través de cuestionarios en papel, cumplimentados por los padres de los participantes. Se recogieron datos sociodemográficos y del estilo de vida, antecedentes familiares y personales.
La información acerca del consumo de lácteos se recogió mediante un cuestionario semicuantitativo de frecuencia de consumo de alimentos (CFCA) de 151 alimentos divididos en diez categorías (lácteos; huevos, carnes y pescados; verduras; frutas; legumbres y cereales; aceites y grasas; golosinas y snacks; bebidas; bollería y pastelería, y miscelánea). Cada participante debía referir el consumo medio de cada uno de los alimentos a lo largo del año previo, eligiendo una de las nueve opciones de respuesta, desde nunca o casi nuca, hasta más de seis veces al día.
Se analizó el efecto del consumo de leches, quesos y yogures por separado, y del conjunto, definiendo la variable “lácteos” como la suma de las tres anteriores. La unidad de consumo era raciones/día. Como se especificaba en los cuestionarios se estableció una ración de leche como el equivalente a 200 ml, y las raciones de queso y yogur como 30 g y 125 g respectivamente.
La variable “leches” incluía el consumo de leche entera, semidesnatada, desnatada, de crecimiento, enriquecidas con calcio, enriquecidas con vitaminas y batidos de leche. No se incluyeron las leches sin lactosa ni las bebidas vegetales. La variable “quesos” incluía el consumo de queso en lonchas, en porciones, queso blanco, y otros quesos. Por último, la variable “yogures” incluía el consumo de cualquier tipo de yogur, incluidos los yogures de sabores, con trozos de frutas, las cuajadas, las natillas, los lácteos a base de queso fresco y las bebidas fermentadas con L. casei inmunitas.
Para estudiar el efecto del consumo de leches, yogures, quesos y productos lácteos en conjunto, definimos dos categorías a partir de la mediana de consumo, estableciendo dos grupos de participantes según estuviera el consumo por encima o por debajo de la mediana (lácteos en conjunto, cuatro raciones de lácteos/día; leches, 2,50 raciones/día; quesos, 0,40 raciones/día, y yogures, 1,30 raciones/día). El grupo de menor consumo fue utilizado como referencia.
Información sobre el desenlace
Se preguntó en los cuestionarios en papel si el participante había sido diagnosticado por un médico de una serie de enfermedades, incluyendo otitis media aguda (OMA), sinusitis, mastoiditis y neumonía. Se evaluaron estas enfermedades en concreto porque tienen un mecanismo fisiopatológico común, que incluye el incremento de la secreción mucosa en las vías respiratorias y adyacentes. Se evaluaron estas patologías ya que uno de los efectos negativos atribuidos al consumo de productos lácteos es que aumentan el moco y las secreciones respiratorias en los niños y secundariamente las infecciones otorrinolaringológicas y respiratorias. A pesar de ser una patología muy prevalente, no se incluyeron los catarros de vías altas (CVA) porque muchos de los episodios de CVA son leves y no reciben atención médica, por lo que resultan más difícil de cuantificar el número de episodios de CVA que padece un niño en edad escolar, lo que dificulta un registro válido. Tampoco se incluyeron en el estudio niños con patología de base como fibrosis quística, discinesia ciliar, bronquiectasias, alergias o inmunodeficiencias, que pudieran cursar con un aumento de las secreciones respiratorias.
Otras variables a estudio
Se recogió información acerca del peso, la talla y el perímetro de cintura del participante. El índice de masa corporal (IMC) se calculó dividiendo el peso (kg) entre el cuadrado de la talla (m2), y el índice-cintura altura (WtHr) se calculó como el cociente entre la circunferencia de la cintura (cm) y la altura (cm). Tanto el IMC como el WtHr se correlacionan bien con la adiposidad15. También se recogió información acerca de la lactancia materna. Se empleó el método de imputación simple para completar los datos faltantes.
Análisis estadístico
Utilizamos el test de t de Student para comparar variables cuantitativas y el test de χ2, o el test exacto de Fisher cuando era necesario, para la comparación de proporciones.
Para estudiar la asociación entre el consumo de lácteos y las infecciones analizadas se realizó una regresión logística multivariable ajustada por sexo, edad, IMC, antecedentes de lactancia materna e ingesta energética total. La categoría de menor consumo fue la categoría de referencia en todos los análisis. A fin de lograr un ajuste más fino por ingesta energética total, se realizó un análisis adicional en el que el consumo de lácteos se ajustó por energía total utilizando el método de los residuales.
El análisis estadístico se realizó con el programa STATA12.0®. Los test se realizaron con un planteamiento bilateral y se consideraron significativos valores de p <0,05.
Aspectos éticos
Los padres o representantes legales de todos los participantes firmaron un consentimiento informado. El estudio fue aprobado por el Comité Ético de Investigación Clínica de Navarra y por el Comité de Ética de la Investigación de la Universidad de Navarra (España).
RESULTADOS
La muestra para el análisis final incluía un total de 169 niños (el 56,2% niñas) nacidos entre 2008 y 2010 (edad media, 6,1 años; desviación estándar [DE], 0,92) (Tabla 1). El IMC medio era de 15,77 (DE: 1,71) y el WtHr medio era de 0,47 (DE: 0,05). La ingesta energética total era significativamente mayor y el WtHr significativamente menor en el grupo de mayor consumo de lácteos en comparación con el grupo de bajo consumo. No se encontraron otras diferencias significativas entre los grupos para el resto de las variables estudiadas.
| Tabla 1. Características sociodemográficas y antropométricas de los participantes en función del consumo de lácteos totales | ||||
|---|---|---|---|---|
| Bajo consumo de lácteos: ≤4 raciones/día (n = 85) | Alto consumo de lácteos: >4 raciones/día (n = 84) | Total (n = 169) | p | |
| Edad (años) | 6 (0,9) | 6,1 (0,9) | 6,1 (0,9) | 0,31 |
| Sexo (mujeres) | 53 (62,3%) | 42 (50%) | 95 (56,2%) | 0,11 |
| Peso (kg) | 21,6 (4) | 22 (3,4) | 21,8 (3,7) | 0,51 |
| IMC (kg/m2) | 15,9 (1,9) | 15,6 (1,3) | 15,8 (1,6) | 0,17 |
| Z score IMC | -0,1 (0,9) | -0,2 (0,7) | -0,1 (0,8) | 0,38 |
| Índice cintura-cadera (WtHr) | 0,48 (0,01) | 0,46 (0,01) | 0,47 (0,004) | 0,03 |
| Ingesta energética (kcal/día) | 1690,3 (414,7) | 2011,2 (405,6) | 1849,8 (439,5) | <0,01 |
| Lactancia materna | 72 (85,7%) | 68 (78,3%) | 137 (82%) | 0,21 |
| Número de hermanos | 2,7 (1,9) | 3,1 (1,8) | 2,9 (1,8) | 0,14 |

Encontramos un consumo medio de lácteos de 4,11 raciones/día, (rango 0,29-9,93 raciones/día). El consumo medio de leche era de 2 raciones/día (rango 0-6,20), el de quesos, de 0,62 raciones/día (rango 0-5,43 raciones/día) y el de yogures, de 1,49 raciones día (rango 0-8,57 raciones/día). Doce niños referían no consumir leche de vaca en absoluto.
En relación con el desenlace, 58 niños de la muestra (el 34,3%) referían haber sido diagnosticado por un médico de alguna de las enfermedades de interés, concretamente OMA (25,4%), sinusitis (1,2%) y neumonía (12,4%).
A pesar de que las estimaciones puntuales, tanto en el análisis crudo como en los modelos ajustados, apuntaban a una posible asociación inversa, no se encontró ninguna asociación significativa entre el consumo de lácteos totales y las enfermedades analizadas en su conjunto (Tabla 2).
| Tabla 2. Odds ratio (IC 95) para el consumo de lácteos y las enfermedades respiratorias | ||
|---|---|---|
| Bajo consumo de lácteos: ≤4 raciones/día (n = 85) | Alto consumo de lácteos: >4 raciones/día (n = 84) | |
| Modelo crudo | 1,00 (Ref.) | 0,92 (0,49 a 1,73) |
| Modelo ajustado por edad y sexo | 1,00 (Ref.) | 0,85 (0,44 a 1,63) |
| Modelo multivariable* | 1,00 (Ref.) | 0,78 (0,39 a 1,59) |
| Modelo multivariable ajustado por el método de los residuales | 1,00 (Ref.) | 0,85 (0,44 a 1,64) |

El modelo ajustado mostró una reducción relativa del riesgo del 15% en el grupo de mayor consumo de lácteos (>4 raciones/día), si bien las diferencias encontradas entre los grupos no resultaron estadísticamente significativas. En relación con cada uno de los tres tipos de lácteos analizados por separado (leches, yogures y quesos) (Fig. 1), encontramos que un mayor consumo de quesos se asociaba con un riesgo significativamente menor de enfermedades en conjunto (odds ratio [OR]: 0,50; intervalo de confianza del 95% [IC 95]: 0,26 a 0,98]). Sin embargo, no se observó ninguna asociación significativa para las leches (OR: 1,02; IC 95: 0,53 a 1,97) ni para los yogures (OR: 0,91; IC 95: 0,47 a 1,75).
| Figura 1. Odds ratio e IC 95 del riesgo de infecciones respiratorias en general, y de neumonías y otitis media en particular, asociado al consumo de los diferentes tipos de lácteos (alto consumo frente a bajo consumo) |
|---|
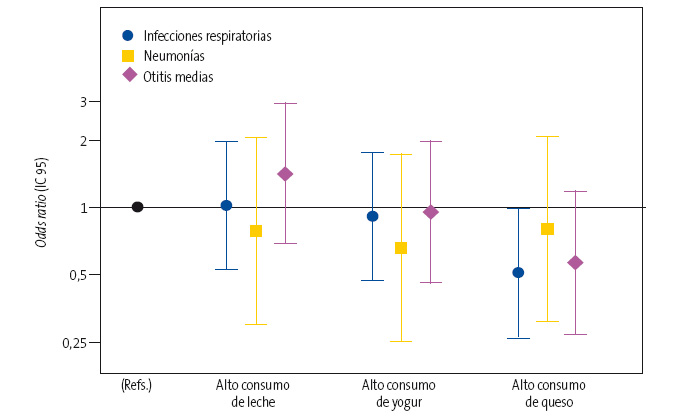 |

De la misma manera, en un análisis de las enfermedades infecciosas por separado, a pesar de que las estimaciones puntuales sugerían una asociación inversa del consumo de yogures y queso con la neumonía (OR: 0,66 [IC 95: 0,25 a 1,73] y OR: 0,81 [IC 95: 0,31 a 2,07], respectivamente) y la OMA (OR: 0,95; [IC 95: 0,46 a 1,96] y OR: 0,57 [IC 95: 0,27 a 1,17] respectivamente), no se encontró ninguna asociación estadísticamente significativa en el análisis multivariable.
DISCUSIÓN
Analizamos si un mayor consumo de lácteos y derivados se asociaba con un mayor riesgo de ciertas enfermedades infecciosas en la infancia (OMA, sinusitis y neumonía) en un estudio transversal con una muestra de 169 niños, y obtuvimos resultados no significativos que no apoyan esa asociación.
Los niños de la muestra referían un consumo medio de 4,11 raciones/día de lácteos (leche y derivados), lo que representa el límite alto de la recomendación para este rango etario. El consumo medio de leche era de 2 raciones/día, lo que equivale a 400 ml, y refleja también un consumo medio superior al recomendado para niños de esa edad9. Es importante señalar que 12 participantes (7%) referían no consumir leche de vaca, un alimento que ha demostrado ser importante en la dieta del niño en fase escolar4,16.
Se encontró una mayor ingesta energética total en el grupo que refería un mayor consumo elevado de lácteos (leche y derivados). Sin embargo, el resto de las variables, incluidas las medidas antropométricas, no eran significativamente diferentes entre los grupos de comparación. El z‑score calculado para los niños de la muestra (z = -0,1) refleja que los participantes tenían un IMC acorde al esperado para su edad y sexo.
La falta de resultados que sugieran una asociación entre el consumo de lácteos en general y las infecciones estudiadas son consistentes con otras investigaciones2,12,13,17. En el análisis de cada uno de los derivados lácteos por separado encontramos estimaciones puntuales de OR que sugerían una posible asociación inversa para los yogures y los quesos. Más concretamente, el grupo que refería un mayor consumo de quesos (>0,42 raciones/día) presentaba una reducción del 50% (IC 95: 2 a 74) del riesgo de infecciones respiratorias. Teniendo en cuenta el tamaño muestral y la falta de evidencia científica a este respecto, estos hallazgos deben interpretarse con cautela. Son necesarios nuevos estudios con muestras de mayor tamaño y un diseño apropiado que repliquen estos resultados antes de concluir la existencia de una verdadera asociación biológica entre el consumo de quesos y las enfermedades respiratorias.
La amplia variabilidad en el consumo de lácteos y la recogida exhaustiva de información representan dos fortalezas de este estudio. Sin embargo, es importante señalar algunas limitaciones. La participación en SENDO es voluntaria y por tanto se trata de una muestra no representativa: ni los datos del consumo de lácteos ni las prevalencias de las enfermedades analizadas son generalizables, pero el diseño del estudio sí permite buscar asociaciones entre el consumo de determinados alimentos y determinadas patologías. La muestra está compuesta en su mayoría por niños de raza caucásica procedentes de familias con un elevado nivel sociocultural (>65% de los padres de los participantes tienen titulación universitaria o superior). Aunque son necesarios nuevos y mejores estudios que repliquen estos resultados en poblaciones más diversas, los mecanismos fisiopatológicos que podrían explicar las asociaciones estudiadas no parecen específicos de una raza ni de un nivel socioeconómico concretos. En los estudios de cohortes, los aspectos más importantes son la validez de los datos autorreferidos y el compromiso de los participantes durante el seguimiento. Existen importantes estudios de cohortes que utilizan muestras muy concretas, como enfermeras (en el estudio Nurses’ Health Study en Boston) o graduados universitarios (estudio SUN en Navarra), que no son representativos de la población general18.
Es cierto que las enfermedades estudiadas son ampliamente diversas en lo que a etiología y epidemiología se refiere, pero se optó por un análisis conjunto porque todas ellas cursan con un aumento de la producción de mucosidad, que es el factor que se ha sugerido que podría estar asociado con el consumo de lácteos, y a pesar de contar con un número elevado de infecciones respiratorias (34%), es posible que el tamaño muestral haya resultado en una falta de potencia estadística. Además, aunque la utilización de información autodeclarada ha sido validada en múltiples estudios en poblaciones pediátricas19,20, este tipo de estudios son susceptibles de un sesgo de información diferencial, que tienden a sesgar la medida de asociación hacia el valor nulo. Por último, el carácter observacional del estudio no permite eliminar por completo la posibilidad de la confusión residual por variables que no se han tenido en cuenta o no se han controlado adecuadamente.
En conclusión, este estudio no encontró una asociación entre un mayor consumo de lácteos y un aumento de las infecciones analizadas en conjunto ni por separado (OMA y neumonías). Estos resultados no apoyan la eliminación o sustitución de la leche o los derivados lácteos de la dieta de los niños con la intención de disminuir las infecciones respiratorias y otorrinolaringológicas. La leche continúa siendo un alimento importante en la alimentación del niño en edad escolar.
Son necesarios nuevos estudios con diseño apropiado y seguimiento prolongado que repliquen estos resultados.
CONFLICTO DE INTERESES
Los autores declaran no presentar conflictos de intereses en relación con la preparación y publicación de este artículo.
FUENTES DE FINANCIACIÓN
Ayudas a la Investigación Ignacio H. De Larramendi de la Fundación MAPFRE 2015 (cantidad total aportada de 15 000 euros para el desarrollo del proyecto piloto SENDO 2015 y para el inicio de la cohorte SENDO).
Beca “Jóvenes Investigadores” de la Sociedad Española de Neumología Pediátrica 2016 (beca de 5000 euros para el proyecto de investigación dentro de SENDO sobre el efecto del consumo de productos lácteos en niños en edad escolar).
ABREVIATURAS
CFCA: cuestionario semicuantitativo de frecuencia de consumo de alimentos • CVA: catarros de vías altas • DE: desviación estándar • IC 95: intervalo de confianza del 95% • IMC: índice de masa corporal • OMA: otitis media aguda • OR: odds ratio • PLV: proteínas de leche de vaca • SENDO: Seguimiento de Escolares Navarros para un Desarrollo Óptimo • SNS-O: Servicio Navarro de Salud-Osasunbidea • WtHr: índice-cintura altura.
AGRADECIMIENTOS
Nuestro agradecimiento a la fundación MAPFRE y a la Sociedad Española de Neumología Infantil. El proyecto SENDO se ha llevado a cabo gracias al trabajo conjunto de la Universidad de Navarra y Atención Primaria del Servicio Navarro de Salud-Osasunbidea. Por ello, queremos agradecer la colaboración de todos los pediatras de Atención Primaria del Servicio Navarro de Salud-Osasunbidea y del Complejo Hospitalario de Navarra y a los investigadores de la Universidad de Navarra. Por último, muchas gracias a todos los participantes y sus familias por su buena disposición a colaborar en el proyecto SENDO.
BIBLIOGRAFÍA
- Woods RK, Wiener JM, Abramson M, Thien F, Walters EH. Patient’s perceptions of food induced asthma. Aust N Z J Med. 1996;26:504-12.
- Lee C, Dozor AJ. Do you believe milk makes mucus? Arch Pediatr Adolesc Med. 2004;158:601-3.
- Thiara G, Goldman RD. Milk consumption and mucus production in children with asthma. Can Fam Physician. 2012;58:165-6.
- Navas López VM, Sierra Salinas C. Errores y mitos en la alimentación infantil. En: Rivero Urgell M, Moreno Aznar LA, Dalmau Serra J. Libro blanco de la nutrición infantil en España. Zaragoza: Prensas de la Universidad de Zaragoza; 2015. p. 131-7.
- MacLellan D, Taylor J, Wood K. Food intake and academic performance among adolescents. Can J Diet Pract Res. 2008;69:141-4.
- Lanou AJ, Berkow SE, Barnard ND. Calcium, dietary products, and bone health in children and young adults: a re-evaluation of the evidence. Pediatrics. 2005;115:736-43.
- Huncharek M, Muscat J, Kupelnick B. Impact of dairy products and dietary calcium on bone-mineral content in children: results of meta-analysis. Bone. 2008;43:312-21.
- Reid IR, Ames R, Mason B, Bolland MJ, Bacon CJ, Reid HE, et al. Effects of calcium supplementation on lipids, blood pressure, and body composition in healthy older men: a randomized controlled trial. Am J Clin Nutr. 2010;91:131-9.
- Moreiras O, Carbajal A, Cabrera L, Cuadrado C. Tablas de composición de alimentos. Guía de prácticas. Madrid: Ediciones Pirámide; 2016.
- Mitchell EA, Stewart AW, Clayton T, Asher MI, Ellwood P, Mackay R, et al. Cross-sectional survey of risk factors for asthma in 6-7-year-old children in New Zealand: International Study of Asthma and Allergy in Childhood Phase Three. J Paediatr Child Health. 2009;45:375-83.
- Lumia M, Takkinen HM, Luukkainen P, Kaila M, Lehtinen-Jacks S, Nwaru B, et al. Food consumption and risk of childhood asthma. Pediatr Allergy Immunol. 2015;26:789-96.
- Arney WK, Pinnock CB. The milk mucus belief: sensations associated with the belief and characteristics of believers. Appetite. 1993;20:53-60.
- Pinnock CB, Arney WK. The milk-mucus belief: sensory analysis comparing cow’s milk and a soy placebo. Appetite. 1993;20:61-70.
- Ortega RM, Navia B, López-Sobaler AM, Aparicio A. Ingestas diarias recomendadas de energía y nutrientes para la población española. Madrid: Universidad Complutense de Madrid; 2014.
- Martín Calvo N, Moreno Galarraga L, Martínez González MA. Association between body mass index, waist-to-height ratio and adiposity in children: a systematic review and meta-analysis. Nutrients. 2016;8:512.
- Pastor Martín MR. Programación de menús infantiles. En: Rivero Urgell M, Moreno Aznar L A, Dalmau Serra J. Libro blanco de la nutrición infantil en España. Zaragoza: Prensas de la Universidad de Zaragoza; 2015. p. 325-31.
- Wüthrich B, Schmid A, Walther B, Sieber R. Milk consumption does not lead to mucus production or occurrence of asthma. J Am Coll Nutr. 2005;24:547-55.
- Bes Rastrollo M, Martínez González MA. Ventajas y limitaciones de los grandes estudios epidemiológicos de seguimiento en nutrición. Endocrinol Nutr. 2006;53:479-83.
- Martín Moreno JM, Boyle P, Gorgojo L, Mainsonneuve P, Fernández Rodríguez JC, Salvini S, Willet WC. Development and validation of a food frequency questionnaire in Spain. Int J Epidemiol. 1993;22:512-9.
- Merson B, Pezdek K, Saywitz K. A meta-analysis of children’s self-reports of dietary intake. Psychol Health. 2017;32:186-203.





