Vol. 15 - Num. 59
Documento de consenso
Documento de consenso sobre etiología, diagnóstico y tratamiento de la sinusitis
L Martínez Camposa, R Albañil Ballesterosb, J de la Flor Bruc, Roi Piñeiro Pérezd, J Cerverae, Fernando Baquero Artigaof, Santiago Alfayate Miguélezg, F Moraga Llopf, MJ Cilleruelo Ortegaa, Cristina Calvo Reyf
aSociedad Española de Infectología Pediátrica (SEIP).
bAsociación Española de Pediatría de Atención Primaria (AEPap).
ccSociedad Española de Pediatría Extrahospitalaria y de Atención Primaria (SEPEAP).
dServicio de Pediatría. Hospital Universitario General de Villalba. Collado Villalba. Madrid. España.
eSociedad Española de Otorrinolaringología Pediátrica (SEOP).
fSociedad Española de Infectología Pediátrica (SEIP). España.
gSección de Infectología Pediátrica. Hospital Clínico Universitario Virgen de la Arrixaca. Murcia. España.
Cómo citar este artículo: Martínez Campos L, Albañil Ballesteros R, de la Flor Bru J, Piñeiro Pérez R, Cervera J, Baquero Artigao F, et al. Documento de consenso sobre etiología, diagnóstico y tratamiento de la sinusitis. Rev Pediatr Aten Primaria. 2013;15:203-18.
Publicado en Internet: 29-08-2013 - Número de visitas: 274358
Resumen
Presentamos el Documento de consenso sobre sinusitis de la Sociedad de Infectología Pediátrica (SEIP), la Asociación Española de Pediatría de Atención Primaria (AEPap), la Sociedad Española de Pediatría Extrahospitalaria y de Atención Primaria (SEPEAP) y la Sociedad Española de Otrorrinolaringología Pediátrica (SEOP).
La sinusitis es una enfermedad de diagnóstico difícil, a menudo no reconocida. Se analiza la etiología, la clínica y los criterios diagnósticos más aceptados, y se realizan recomendaciones terapéuticas acordes con la situación epidemiológica actual. Se propone la amoxicilina por vía oral como tratamiento antibiótico de elección en dosis de 80 mg/kg/día repartidas cada 8 horas. Se indican tratamientos alternativos en casos especiales y en ausencia de eficacia de la amoxicilina. Se revisan las principales complicaciones de esta entidad.
Palabras clave
● Amoxicilina ● Diagnóstico ● Rinosinusitis ● Sinusitis ● TratamientoNota:
Artículo publicado simultáneamente en Anales de Pediatría: http://dx.doi.org/10.1016/j.anpedi.2013.04.027
INTRODUCCIÓN
Se define sinusitis como la inflamación de uno o más senos paranasales que ocurre habitualmente como complicación de una infección respiratoria viral de vías aéreas superiores. Cuando la duración del cuadro es superior a diez días, se presupone sobreinfección bacteriana. En general se diagnostica por la clínica y, aunque suele ser una enfermedad autolimitada, llega a ser la tercera causa de prescripción de antibióticos en Atención Primaria (tras la otitis y la amigdalitis), a pesar de ser un proceso infradiagnosticado y a menudo no registrado.
Las controversias sobre la sinusitis abarcan su definición e identificación, la implicación de infecciones virales o bacterianas y factores no infecciosos en su evolución, el diagnóstico clínico frente a la utilidad de pruebas complementarias y el manejo con antibióticos y otras medidas coadyuvantes1.
Según la sistemática de otros documentos de consenso, se ha añadido la fuerza de la recomendación (A: buena evidencia, B: moderada evidencia, C: poca evidencia) y la calidad de la evidencia científica (I: ensayos controlados aleatorizados, II: estudios bien diseñados pero no aleatorizados, III: opiniones de expertos basadas en experiencia clínica o series de casos) de las medidas propuestas, siguiendo el sistema de calificación de la Infectious Disease Society of America.
DEFINICIONES
La Academia Americana de Pediatría define en 2001 estos procesos como2:
- Sinusitis aguda bacteriana: infección bacteriana de los senos paranasales, de duración inferior a 30 días y con resolución completa de los síntomas.
- Sinusitis subaguda: infección bacteriana de los senos paranasales de duración entre 30 y 90 días. Presenta una microbiología similar a la aguda.
- Sinusitis aguda recurrente: episodios de infección bacteriana que duran menos de 30 días y están separados entre sí al menos 10 días, durante los cuales el paciente está asintomático. El paciente debe presentar 3 episodios de sinusitis aguda en 6 meses, o 4 en 12 meses.
- Sinusitis crónica: episodios de inflamación que duran más de 90 días. Los pacientes mantienen síntomas respiratorios (tos, rinorrea, obstrucción nasal) residuales persistentes.
- Sinusitis crónica con episodios de sinusitis aguda bacteriana: los pacientes desarrollan nuevos síntomas que se resuelven con antibiótico, mientras que los previos siguen persistiendo.
En las últimas guías internacionales de práctica clínica se ha adoptado por consenso el término "rinosinusitis" para referirse a la inflamación aguda, subaguda o crónica con independiencia de su causa, puesto que la mucosa rinosinusal es continua y no hay afectación sinusal exclusiva sin afectación previa o concomitante de la mucosa nasal. De todas formas, por el momento se sigue utilizando indistintamente el antiguo término "sinusitis" para referirse a ambas entidades3.
EPIDEMIOLOGÍA
Según estadísticas estadounidenses3, la rinosinusitis aguda afecta aproximadamente a 31 millones de pacientes (adultos y niños) por año, con implicaciones en la calidad de vida y la utilización de recursos sanitarios, y es motivo de una alta prescripción de fármacos. Se calcula que un 1% de los niños padecerá sinusitis cada año, y que ello ocasionará tanto un gasto importante en salud como en consumo de antibióticos.Puesto que la neumatización de algunos senos está presente desde el nacimiento, puede producirse sinusitis desde la época de lactante, pero es un diagnóstico que con frecuencia no consideran los pediatras en los niños menores de un año4.
En España no contamos con estadísticas reales relativas a la incidencia de rinosinusitis, pero si inferimos que la situación es similar a la de otros países industrializados, y teniendo en cuenta que los niños presentarán unas 3 a 8 infecciones respiratorias al año, podemos prever que su impacto en salud y en prescripción antibiótica no es desdeñable5.
FISIOPATOLOGÍA
Debemos considerar una serie de aspectos como son: a) la anatomía y el desarrollo de los senos paranasales en los niños, b) el papel de la mucosa nasal en las infecciones virales/bacterianas y c) los factores predisponentes o agravantes.
Los senos paranasales en niños
Los senos paranasales se dividen en 5 grupos según su localización y vía de drenaje: los senos etmoidales anterior y posterior, que drenan en los meatos medio y superior, respectivamente; los dos senos maxilares, que drenan en el meato medio, y el seno frontal6:
- Los senos etmoidales son visibles al nacimiento, crecen rápidamente hasta los 7 años y completan su crecimiento a los 15-16 años.
- Los senos maxilares están neumatizados al nacer, con un volumen de 2 ml a los dos años de edad, alcanzan unos 10 ml a los 9 años y finalizan su crecimiento a los 15 años.
- Los senos frontales son indistinguibles de las celdas etmoidales anteriores y crecen tan lentamente que no pueden ser identificados anatómicamente antes del año de edad. Después del cuarto año de edad, comienzan a agrandarse y a la edad de 6 años pueden identificarse radiológicamente en un 20-30% de los niños. Continúan creciendo en la adolescencia y a los 12 años más del 85% de los niños los muestran neumatizados en la tomografía computarizada (TC).
- El seno esfenoidal es apenas una evaginación del receso esfenoetmoidal. A los 7 años de edad, se ha extendido posteriormente hasta la silla turca y en un 85% de los pacientes está neumatizado en la TC a los 8 años de edad, completando su crecimiento a los 15 años1.
La mucosa rinosinusal
La mucosa nasal y de los senos paranasales tiene funciones específicas, como son el filtro y calentamiento del aire inspirado y la inmunorrespuesta a alérgenos, contaminantes y otras partículas para proteger la delicada estructura de la vía aérea inferior. Está demostrada la implicación de la mucosa sinusal en las infecciones virales de las vías aéreas superiores, que en la mayor parte de los casos se resuelven espontáneamente (rinosinusitis viral aguda), pero en algunos se produce una obstrucción del ostium, con absorción del oxígeno de la cavidad por presión negativa que favorece la aspiración del moco nasofaríngeo rico en bacterias (rinosinusitis posviral aguda), contaminando los senos paranasales (estériles en condiciones normales), que si no es eliminado por el aparato mucociliar se produce multiplicación bacteriana, desarrollándose una infección bacteriana de la mucosa sinusal (rinosinusitis bacteriana aguda), lo cual ocurre en un 6-10% de los casos1,7,8.
Factores predisponentes o agravantes
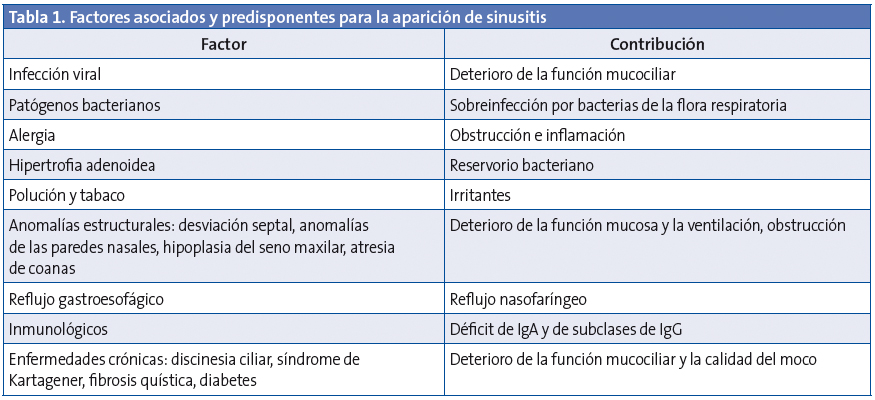
La inflamación de la mucosa rinosinusal se produce por interacción entre la noxa atacante (infecciosa o no), los factores defensivos locales y el sistema del huésped, y presenta algunos factores predisponentes1,9,10(Tabla 1).
ETIOLOGÍA
Los factores que van a influir en el desarrollo de la rinosinusits bacteriana incluyen, entre otros, la colonización nasofaríngea, el estado de vacunación y los tratamientos antibióticos previos.
Flora normal de la nasofaringe
En la nariz hay una colonización con flora polimicrobiana que en estudios en niños, se ha observado que incluye Streptococcus pneumoniae (S. pneumoniae) (50-60%), Haemophilus influenzae (H. influenzae) no tipable (40-68%), Moraxella catarrhalis (M. catarrhalis)(34-50%) y, en menor medida Streptococcus viridans, Streptococcus pyogenes (S. pyogenes) y Neisseria spp.11. Los porcentajes son más altos en los niños adenoamigdalectomizados12. La presencia de esta flora en niños asintomáticos reafirma la poca fiabilidad de los cultivos de meato en el diagnóstico etiológico.
Bacterias implicadas en la rinosinusitis aguda
La mayor parte de las infecciones sinusales son virales y solo una pequeña proporción desarrolla una infección bacteriana secundaria5.Rinovirus, influenza y parainfluenza son las causas más comunes de rinosunisitis aguda.Los senos paranasales son estériles en condiciones fisiológicas9, por lo que los cultivos de muestras de senos paranasales mediante punción serían los más adecuados para el diagnóstico etiológico. En los escasos estudios realizados en niños mediante esta técnica, S. pneumoniae se aísla en un 35-42%, H. influenzae en un 21-28%, M. catharrralis en un 21-28%, S. pyogenes en un 3-7%, y microorganismos anaerobios (en los procesos crónicos y odontogénicos) en un 3-7%13-16.A su vez, es posible la coinfección bacteriana y la implicación de diferentes bacterias en la enfermedad polisinusal17.
Impacto de la vacunación antineumocócica
Hay trabajos que han valorado las implicaciones microbiológicas que la implantación de las vacunas antineumocócicas conjugadas ha tenido en la etiología de las infecciones respiratorias:
- Se observa en niños una disminución de la colonización nasal y orofaríngea por S. pneumoniae con un aumento relativo de la presencia de H. influenzae no tipificable18.
- La inmunidad de grupo debida a la vacunación se manifiesta en la etiología de la sinusitis en adultos, con una disminución del 10% en los aislamientos de S. pneumoniae, y un cambio en los serotipos detectados, así como un aumento del 6% de H. influenzae19.
- Es necesario estudiar el efecto que tendrá la implantación de la vacuna antineumocócica conjugada 13-valente sobre la rinosinusitis, ya que los estudios publicados se refieren a la heptavalente.
Resistencia antibiótica
La prevalencia de resistencia de S. pneumoniae a la penicilina se encuentra entre el 10 y el 30%, y a macrólidos en torno al 25%, con variaciones geográficas y modificaciones secundarias a la implantación de las vacunas, en el sentido de una disminución de las resistencias a las penicilinas tras la introducción de la vacuna antineumocócica conjugada 13-valente (esta vacuna no está incluida en el calendario vacunal unificado presentado por el Ministerio de Sanidad, pero sí lo está en las recomendacionesdel Comité Asesor de Vacunas de la Asociación Española de Pediatría20-25. La resistencia a los macrólidos ha experimentado un descenso en su porcentaje del 26,4 al 20%, mientras que la resistencia al levofloxacino ha pasado del 0,1 al 1,3% (2007), ambas asociadas al uso de estos antibióticos (disminución y aumento, respectivamente). La producción de betalactamasas por parte de H. influenzae ha decrecido en los últimos años26, del 33 al 17,4%, tendencia de descenso que se mantiene, con una aparición paulatina de cepas resistentes a la ampicilina por un mecanismo diferente de la producción de betalactamasas27. Del 90 al 100% de M. catarrhalis siguen siendo productoras de betalactamasas28.
CLÍNICA
Los síntomas más frecuentes de rinosinusitis bacteriana son la congestión nasal, habitualmente bilateral, la rinorrea de cualquier tipo, consistencia y color, y la tos persistente, que puede empeorar por la noche. Pueden existir vómitos ocasionados por la rinorrea posterior29. Otros síntomas son dolor facial o sensación de presión, que puede localizarse a nivel dental, en la mandíbula superior, ojos, frente o hemicara, y aumentar al inclinar la cabeza hacia delante (siendo el dolor en general menos prevalente en los niños)30. También puede haber hiposmia o anosmia, e inflamación periocular. En los niños más pequeños puede haber síntomas más inespecíficos, como irritabilidad o poco apetito31. En preescolares puede percibirse halitosis, otalgia y odinofagia así como sibilancias. La cefalea podría ser el único síntoma en algunos pacientes (esfenoiditis), pero tanto esta como el dolor facial aislados sin otros síntomas no suelen ser datos específicos de sinusitis.
Los síntomas que harían sospechar la aparición de complicaciones son edema periorbital, las alteraciones de la motilidad ocular, reaparición de fiebre, cefalea importante, vómitos, alteración del estado mental, convulsiones, focalidad neurológica y los síntomas de hipertensión intracraneal29,32
DIAGNÓSTICO
El diagnóstico de confirmación de una rinosinusitis bacteriana es el aislamiento de ≥104 unidades formadoras de colonias en una muestra obtenida mediante punción del seno33, pero este procedimiento no se realiza, ni debe realizarse, de rutina en la práctica clínica.
El diagnóstico de sinusitis bacteriana debe realizarse según criterios clínicos, y reservar la realización de pruebas complementarias ante la sospecha de complicaciones, mala respuesta al tratamiento, procesos recurrentes o situaciones clínicas especiales como inmunodepresión o enfermedad grave de base.
Para el diagnóstico se definen 3 formas de presentación34:
- Sintomatología catarral prolongada: congestión o rinorrea, tos o ambos, que persisten sin mejoría más de 10 y menos de 30 días (IIB). La rinorrea puede ser acuosa, mucosa o purulenta y la tos seca o productiva, y es frecuente que empeore por la noche. Esta sería la forma de presentación de la mayoría de sinusitis agudas bacterianas.
- Inicio brusco de síntomas más graves, fundamentalmente fiebre alta (≥39 °C) que dura más de 3-4 días y rinorrea purulenta (IIB).
- Empeoramiento de los síntomas en la evolución de un catarro común, con aumento de la rinorrea, tos diaria o aparición o reaparición de fiebre, especialmente si este empeoramiento se produce a partir de los 6-7 días de evolución (IIB).
En el 70% de los catarros comunes en los escolares, a los 10 días de evolución, se mantiene algún síntoma, pero se ha producido una mejoría de todos ellos35.Las últimas guías (americanas y europeas) coinciden en que son fundamentalmente la persistencia, la gravedad y el empeoramiento de la sintomatología catarral las claves diagnósticas del proceso. Sin embargo, advierten de la imposibilidad de diferenciar con seguridad por criterios clínicos la rinosinusitis viral de la bacteriana, lo que supone una dificultad a la hora de seleccionar pacientes que podrían recibir tratamiento antibiótico y evaluar los resultados del mismo, sobre todo por la falta de criterios unificados a la hora de incluir a los pacientes en los estudios1,34.
EXPLORACIÓN FÍSICA
No suele ayudar en el diagnóstico, porque los posibles hallazgos pueden estar ausentes, son poco específicos y no diferencian entre etiología viral o bacteriana36. Puede verse la mucosa nasal eritematosa o pálida, rinorrea en las fosas nasales, moco en la pared posterior de faringe y eritema faríngeo y timpánico. Puede observarse inflamación periorbital blanda y no dolorosa. Es posible que haya dolor a la palpación frontal y maxilar, pero la sensibilidad facial es una prueba poco sensible y específica. La presencia de halitosis en ausencia de faringitis, cuerpo extraño o mala higiene dental puede hacer sospechar una sinusitis.
PRUEBAS COMPLEMENTARIAS
No está indicada la realización sistemática de analítica para el diagnóstico de rinosinusitis aguda no complicada.
Estudios de imagen
- La radiología convencional de senos ha sido tradicionalmente una herramienta para el diagnóstico, pero en Pediatría es una prueba complementaria sensible aunque poco específica. Los signos más frecuentemente encontrados, la opacificación de senos y la hipertrofia de la mucosa superior a 4 mm, tienen escaso valor predictivo positivo, puesto que son habituales en niños sanos o con resfriado común, rinosinusitis vírica o rinitis alérgica37. Un 35-50% de niños sanos entre uno y nueve años y hasta el 97% de los pacientes con un cuadro catarral concomitante o reciente presentan falsos positivos38.El nivel hidroaéreo, de mayor especificidad, es un hallazgo poco frecuente37,38. La radiología debería considerarse únicamente en situaciones de fracaso terapéutico o clínica grave con sospecha de complicaciones intracraneales.
- La TC es más fiable, pero también puede estar alterada en niños con cuadro catarral leve y sin criterios clínicos de sinusitis, requiere en muchas ocasiones de sedación y la irradiación necesaria supera a la de la radiología simple. Sin embargo, si se precisa alguna técnica de imagen, es la que ofrece mejor rendimiento diagnóstico39. La TC debe hacerse urgentemente en caso de proptosis, alteración del movimiento ocular o de la visión, cefalea intensa, vómitos repetidos, convulsiones o alteración del sensorio.
- La resonancia magnética (RM) tiene un elevado coste y también requiere frecuentemente de sedación. Define peor que la TC la estructura ósea del complejo osteomeatal, y también presenta alteraciones en cuadros catarrales40, aunque es más sensible en la detección precoz de complicaciones intracraneales, en la diferenciación entre inflamación y tumor y en la sinusitis crónica micótica (muy rara en niños)41.
Podemos concluir que los estudios de imagen no están indicados en el estudio de la sinusitis aguda pediátrica no complicada, y deberían reservarse para el estudio de la sinusitis persistente, recurrente, crónica o ante sospecha de complicación.
Otros exámenes complementarios
- La endoscopia sinusal ha mostrado correlación adecuada con los hallazgos de la TC, pero no es una técnica utilizable de forma rutinaria42.
- La transiluminación o diafanoscopia es muy poco fiable en Pediatría, pues los senos son de pequeño tamaño, y los hallazgos, difíciles de valorar y en cualquier caso aplicables solo a senos maxilares43.
- La ecografía portátil de senos paranasales es mucho más prometedora, pero aún poco conocida y practicada. Es una exploración rápida, simple y de carácter no invasivo. El procedimiento es indoloro, se puede repetir ilimitadamente, es de interpretación sencilla y no irradia al niño. Esta técnica, en manos expertas, ha demostrado una sensibilidad (>86%) y especificidad (>96%) muy superiores a las de la radiología, para determinar la presencia de exudado de los senos maxilares. No obstante, también tiene sus limitaciones: no sirve para el diagnóstico de la sinusitis etmoidal ni esfenoidal, y el coste del utillaje dificulta su incorporación sistemática a la consulta del pediatra44-46.
DIAGNÓSTICO DIFERENCIAL
El diagnóstico diferencial se establece con los siguientes procesos:
- Catarro común y rinitis aguda: suelen ser afebriles o con fiebre de bajo grado y menor duración, y la tos y la rinorrea mejoran desde el quinto o sexto día de evolución. En la sinusitis no se produce esta mejoría, puede haber afectación general y la fiebre, si la hay, así como el resto de síntomas son más intensos y prolongados. Puede ser difícil diferenciar la sinusitis de los procesos catarrales recurrentes, tan frecuentes en los niños, si bien en estos debe haber intervalos libres de síntomas1,29.
- Procesos que cursan con obstrucción, secreción nasal y tos persistente1,47,48. Se detallan en la Tabla 2.

- Afecciones que cursen con dolor facial o craneal, como cefalea tensional, dolor de origen dental, dolor facial neuropático atípico y disfunción temporomandibular49. Ante cuadros recurrentes o evoluciones atípicas deben excluirse factores predisponentesy enfermedades de base (Tabla 1).
CRITERIOS DE DERIVACIÓN
Los criterios de derivación hospitalaria se detallan en la Tabla 3.

COMPLICACIONES DE LA SINUSITIS
Las complicaciones se presentan en el 3,7-11% de las sinusitis agudas bacterianas y se dividen en orbitarias (60-70%), intracraneales (15-20%) y óseas (5-10%) (Tabla 4). La afectación orbitaria se produce más frecuentemente entre los 3 y los 6 años y las complicaciones intracraneales son más habituales en la adolescencia50,51. La complicación más frecuente de la rinosinusitis aguda es la celulitis periorbitaria.
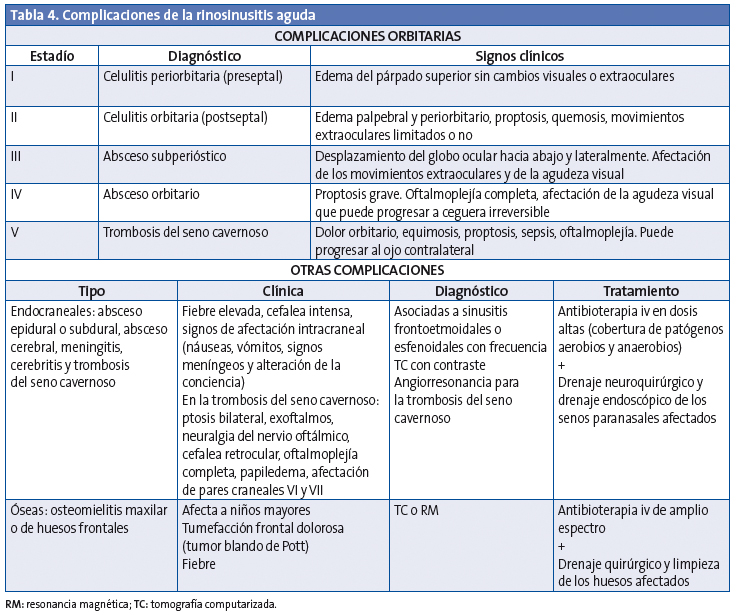
Complicaciones orbitarias
La extensión de la infección a la órbita se produce con facilidad directamente a través de la lámina papirácea, que es muy fina y a veces dehiscente; también se puede extender por vía venosa52. La clasificación de las complicaciones orbitarias fue realizada en 1970 por Chandler et al.53 con un sistema que organiza los modelos típicos de la afectación orbitaria en una progresión lógica de la enfermedad (Tabla 4). Es importante saber que las complicaciones orbitarias en los niños pueden producirse sin dolor.
Celulitis periorbitaria o preseptal
Es la inflamación del párpado y de la conjuntiva, que afecta al tejido anterior al tabique orbitario, y se ve fácilmente en una TC como una inflamación de tejidos blandos. Se produce con frecuencia como complicación de una infección del tracto respiratorio superior, dacriocistitis o infección de la piel, y de una sinusitis54. Cursa con edema palpebral, eritema y fiebre. No se asocia a proptosis, ni hay limitación de movilidad ocular. Normalmente responde bien a tratamiento antibiótico, pero si no se trata precozmente puede extenderse más allá del tabique orbitario. En la mayoría de las situaciones la celulitis preseptal es un diagnóstico clínico y no precisa la realización de una TC para su evaluación55.
Celulitis orbitaria o postseptal
Se desarrolla a medida que los cambios inflamatorios afectan a la órbita, con aparición de edema conjuntival, proptosis y una movilidad ocular reducida y dolorosa56. Esta complicación requiere un tratamiento intensivo con antibioterapia intravenosa, así como excluir la existencia de un absceso subperióstico u orbitario mediante TC. En caso de sospecha de complicación intracraneal, se deberá completar el estudio con una RM.
Absceso subperióstico y orbitario
El absceso subperióstico se forma entre la periórbita y los senos paranasales y está localizado en la parte más externa de los músculos oculares. Los signos clínicos son edema, eritema, equimosis y proptosis, con limitación de la movilidad (oftalmoplejía) y disminución de la agudeza visual57.
El absceso orbitario es intraconal, limitado por los músculos rectos y las membranas que los unen y la cápsula de Tenon. Habitualmente se produce en casos de retraso en el diagnóstico o en pacientes inmunodeprimidos, con una frecuencia que oscila entre el 8 y el 13%58.
Cuando hay un absceso, confirmado con TC, y pérdida visual progresiva o ausencia de mejoría clínica tras 48 horas de tratamiento intravenoso, está indicado el drenaje orbitario59.Debe hacerse un seguimiento oftalmológico de la agudeza visual, y podrá cambiarse a antibioterapia oral cuando el paciente esté afebril más de 48 horas y haya una resolución de los signos y síntomas oftalmológicos.
Los consensos actuales recomiendan que las celulitis preseptales y orbitarias deben tratarse inicialmente con antibioterapia, mientras que los abscesos subperiósticos e intraorbitarios precisan tratamiento quirúrgico, habitualmente mediante endoscopia57. Sin embargo, hay estudios recientes que demuestran buenos resultados con antibióticos intravenosos en niños con abscesos subperiósticos60, siempre que se cumplan las siguientes condiciones: mejoría clínica en 24-48 horas, ausencia de disminución de la agudeza visual, absceso subperióstico pequeño (<0,5 a 1 ml) localizado medialmente, ausencia de afectación sistémica y edad del paciente entre 2 y 4 años 61.
TRATAMIENTO
Tratamiento no antibiótico
La utilización de vitamina C, zinc, equinácea, descongestivos, antihistamínicos sistémicos o mucolíticos no se recomienda en las últimas revisiones, por falta de efectividad y/o potencial toxicidad1,62,63.
Las soluciones salinas iso- o hipertónicas producen una mejoría subjetiva de los síntomas y del aclaramiento mucociliar64-66, mejoran la eliminación de las secreciones y evitan la formación de costras, pero los datos aún son limitados para hacer una recomendación con suficiente nivel de evidencia67.
Los corticoides orales como tratamiento adyuvante a los antibióticos orales son efectivos para el alivio de los síntomas en la sinusitis aguda a corto plazo. Sin embargo, los datos son limitados y no hay estudios de calidad que justifiquen su uso en monoterapia ni como tratamiento adyuvante a la antibioterapia68.
Los corticoides intranasales parecen tener alguna utilidad junto con los antibióticos, sobre todo en estudios realizados en adultos, y podrían ser beneficiosos en niños con rinitis alérgica de base, pero son necesarios más estudios que avalen su verdadera utilidad en la sinusitis pediátrica69.
Tratamiento antibiótico
En 2001, la AAP recomendó el uso de antibióticos en la sinusitis aguda bacteriana, aunque su eficacia en cuanto al control de los síntomas y, sobre todo, con respecto a la prevención de las posibles complicaciones de la enfermedad es aún muy controvertida1,34,70-75. La curación espontánea de la sinusitis aguda no complicada es alta (60-80%), por lo que actualmente la tendencia es recomendar la prescripción de antibióticos únicamente para los casos persistentes o complicados. El grupo de consenso recomienda iniciar tratamiento antibiótico siempre que se cumplan los criterios diagnósticos de sinusitis bacteriana (véase el apartado de diagnóstico), a excepción de los niños que, aun manteniendo síntomas durante al menos 10 días, muestran una evolución clínica favorable. En este supuesto, la actitud sería expectante, con vigilancia clínica y tratamiento sintomático.
Tratamiento de elección
El tratamiento de elección en nuestro medio es amoxicilina76-78, que tiene buena actividad frente neumococo, la bacteria más frecuentemente implicada y la que presenta una tasa más alta de complicaciones. En áreas con altas tasas de vacunación antineumocócica se ha observado una disminución en la colonización nasofaríngea por el neumococo y un aumento de los aislamientos de H. influenzae no tipable y de M. catarrhalis. En esta situación puede utilizarse como alternativa amoxicilina-clavulánico, ya que la mayoría de los aislamientos de M. catarrhalis y el 10-20% de H. influenzae producen betalactamasas. La amoxicilina-clavulánico también se recomienda en sinusitis con riesgo de complicaciones cuando se desee cubrir todas las situaciones: niños menores de 2 años, sinusitis frontales o esfenoidales, sinusitis etmoidales complicadas, pacientes con sintomatología muy intensa o prolongada (más de un mes), pacientes inmunodeprimidos o con enfermedades crónicas, o no respuesta al tratamiento inicial con amoxicilina.
En España, la dosis recomendada de amoxicilina (tanto sola como asociada a clavulánico) es de80-90 mg/kg/día repartida cada 8 horas, ya que las tasas de resistencia del neumococo a la penicilina son superiores al 10%.
Tratamiento alternativo
Las cefalosporinas orales de segunda generación (cefuroxima axetilo), y las cefalosporinas orales de tercera generación (cefpodoxima proxetil y ceftibuteno) y las fluoroquinolonas también han resultado eficaces en diversos estudios, pero los resultados no han sido superiores a los conseguidos con amoxicilina o amoxicilina-clavulánico79,80, por lo que su uso debería restringirse a pacientes con alergia no tipo I a la penicilina. Aunque los macrólidos no son una buena opción terapéutica por su alto porcentaje de resistencias (25-30%), en caso de alergia inmediata o acelerada (tipo I) sin afectación grave, pueden emplearse y preservar otras opciones terapéuticas. Otra opción en estos pacientes con sintomatología leve es la observación estrecha sin antibioterapia. Como última opción, en este grupo especial de niños con alergia tipo I grave a la penicilina y mala respuesta al tratamiento con macrólidos, podría emplearse levofloxacino.
Duración de la antibioterapia
Se recomienda una duración del tratamiento antibiótico entre 7 y 14 días1,32 siendo 10 días la pauta más aconsejada76,77,81,82. Algunos pacientes con respuesta más lenta requieren un tratamiento más largo, y en este caso se recomienda prolongar la antibioterapia hasta 7 días después de la desaparición de los síntomas clínicos. En determinados casos (niños con respuesta parcial) puede prolongarse hasta 3 semanas76,81.
Actitud recomendada en caso de fracaso terapéutico
Con un tratamiento adecuado, en 48-72 horas los niños suelen quedar afebriles, y la tos y la rinorrea disminuyen paulatinamente76,82. Si no es así, deben replantearse el diagnóstico y el tratamiento76,82. Las principales causas de fracaso del tratamiento, una vez asegurado su correcto cumplimiento, son: a) microorganismo resistente al antibiótico utilizado, b) desarrollo de complicaciones, c) etiología no infecciosa (cuerpo extraño intranasal, malformación estructural y alergia) o, excepcionalmente, d) existencia de enfermedades crónicas o inmunodeficiencias. En caso de sospecha de microorganismo resistente, es conveniente modificar la antibioterapia empírica, añadiendo un antimicrobiano eficaz contra bacterias productoras de betalactamasas o contra neumococos con alta resistencia a la penicilina: amoxicilina-clavulánico o incluso cefalosporinas de tercera generación (ceftriaxona intramuscular)76,82.
Criterios de hospitalización y selección de la antibioterapia empírica intravenosa
Los niños con aspecto séptico, afectación del estado general, fracaso persistente del tratamiento oral o con complicaciones (valorable en celulitis preseptal) deben ser hospitalizados y tratados por vía parenteral con alguno de los siguientes antibióticos: amoxicilina-clavulánico, cefotaxima o ceftriaxona76,82.Se recomienda realizar pruebas de imagen para confirmación diagnóstica y valoración por el especialista en Otorrinolaringología (ORL). Ante la sospecha de complicaciones intracraneales con posible presencia de microorganismos anaerobios, debe asociarse cefotaxima con metronidazol. En casos de alergia tipo I a la penicilina, el levofloxacino asociado a metronidazol puede ser una opción en pacientes graves.
Protocolo de tratamiento
- Tratamiento médico no antibiótico:
- Analgesia: recomendado (IA). Ibuprofeno o paracetamol por vía oral a las dosis habituales. El ibuprofeno muestra un perfil de actuación mejor debido a su doble acción analgésica y antiinflamatoria.
- Lavados con suero salino: prueba terapéutica recomendada (IIB). Se trata de un tratamiento barato e inocuo que en algunos estudios se ha mostrado eficaz.
- Corticoterapia intranasal: recomendado en niños con base de rinitis alérgica (IIIC); prueba terapéutica en niños sin base alérgica (IIIC), sobre todo en la opción observación sin antibióticos.
- Mucolíticos, descongestivos y antihistamínicos: no recomendados (IA).
- Observación sin antibióticos: se recomienda no iniciar antibioterapia en los niños que, a pesar de tener sintomatología durante más de 10 días, presentan una clara evolución favorable.
- Tratamiento antibiótico oral: recomendado en el resto de los pacientes (IIB):
- De elección:
- Amoxicilina en dosis de 80-90 mg/kg/día repartida cada 8 horas durante 10 días (IIB).
- En niños menores de dos años, sinusitis esfenoidal o frontal, celulitis preseptal incipiente, inmunocomprometidos o con enfermedad importante de base, sintomatología muy intensa o prolongada (mayor de un mes) y cuando no se objetive respuesta al tratamiento inicial con amoxicilina:
- Amoxicilina-clavulánico (8/1) en dosis de 80-90 mg/kg/día repartida cada 8 horas durante 10 días (IIB).
- En niños con alergia retardada a penicilina (reacción no anafiláctica):
- Cefpodoxima proxetil en dosis de 10 mg/kg/día repartidos cada 12 horas durante 10 días (IIB).
- Ceftibuteno en dosis de 9 mg/kg/día cada 24 horas (máximo 400 mg al día), durante 5-10 días (IIIC).
- Cefuroxima axetilo en dosis de 30 mg/kg/día repartidos cada 12 horas, durante 10 días (IIB).
- En niños con alergia inmediata o acelerada a penicilina (reacción anafiláctica, tipo I):
- Valorar especialmente la posibilidad de vigilancia clínica sin antibioterapia (IIIC).
- En casos no graves, claritromicina 15 mg/kg/día cada 12 horas (IIIC) o azitromicina 10 mg/kg/día repartidos cada 24 horas 3 días, o 10 mg/kg/día el primer día y 5 mg/kg/día cuatro días más.
- En casos graves o si han fracasado los macrólidos, levofloxacino en dosis de 10-20 mg/kg/día repartido cada 12-24 horas durante 10 días (uso off-label) (IIIC).
- En niños con mala tolerancia oral inicial:
- Ceftriaxona intramuscular en dosis de 50 mg/kg/día cada 24 horas durante 1-3 días, seguida de una de las anteriores pautas (en función del caso) hasta completar 10 días (IIIC). La ceftriaxona es un fármaco de dispensación hospitalaria, por lo que el paciente debe ser remitido para valorar su administración.
- De elección:
- Actitud recomendada en caso de fracaso terapéutico tras 48-72 horas de antibioterapia inicial correcta:
- Diagnóstico diferencial:
- Complicaciones.
- Etiología no infecciosa.
- Inmunodeficiencias.
- Valorar pruebas radiológicas si se sospechan complicaciones (IIIC).
- Cambio de antibioterapia oral empírica, en función de la elección inicial (IIB):
- Amoxicilina-clavulánico en dosis de 80-90 mg/kg/día cada 8 horas si se inició tratamiento con amoxicilina (IIB).
- Las cefalosporinas orales (cefuroxima o ceftibuteno) no aportan beneficios sobre amoxicilina-clavulánico por lo que, en este consenso, no se recomiendan en caso de fracaso del tratamiento inicial.
- Levofloxacino en dosis de 10 mg/kg cada 12 horas en niños de 6 meses a 5 años de edad y 10 mg/kg/dosis cada 24 horas en mayores de 5 años (dosis máxima 500 mg/día) (uso off-label) en niños con alergia tipo I a penicilinas (anafilaxia) si no ha sido efectivo el tratamiento con macrólidos (IIIC).
- Los pacientes que no han mejorado con las pautas anteriormente descritas deberían ser remitidos al hospital para recibir ceftriaxona intramuscular.
- Diagnóstico diferencial:
- Criterios de derivación y tratamiento hospitalario:
- Criterios de hospitalización:
- Aspecto séptico.
- Afectación del estado general.
- Fracaso persistente de dos ciclos de tratamiento oral (criterio de valoración hospitalaria con o sin ingreso).
- Complicaciones (con la posible excepción de la celulitis preseptal).
- Entorno familiar de riesgo que no garantice el cumplimiento terapéutico.
- Pruebas radiológicas recomendadas (IIB).
- Valoración por especialistas en ORL y Oftalmología en caso de celulitis orbitaria o periorbitaria (IIIC).
- Tratamiento intravenoso (IIB):
- Amoxicilina-clavulánico en dosis de 100 mg/kg/día repartidas cada 6 horas (IIB).
- Cefotaxima en dosis de 150-200 mg/kg/día repartidas cada 6 u 8 horas (IIB) o ceftriaxona en dosis de 50-100 mg/kg/día cada 12 o 24 horas (IIIC), si el paciente había recibido previamente amoxicilina-clavulánico.
- Levofloxacino en dosis de 10 mg/kg cada 12 horas en niños de 6 meses a 5 años de edad y 10 mg/kg/dosis cada 24 horas en mayores de 5 años (dosis máxima 500 mg/día) (uso off-label) (IIIC), en caso de niños con alergia tipo I a la penicilina.
- En caso de sospecha de complicación intracraneal y en caso de riesgo de microorganismos anaerobios:
- Añadir metronidazol al tratamiento con cefotaxima (o levofloxacino en alérgicos), en dosis de 30 mg/kg/día repartidas cada 6 horas (IIIC).
- En caso de fracaso de antibioterapia intravenosa, debe valorarse la presencia de complicaciones en conjunto con el especialista en ORL y un experto en Infectología pediátrica.
- Criterios de hospitalización:
CONFLICTO DE INTERESES
Los doctores Martínez, Baquero, Calvo, de la Flor, Alfayate, Cilleruelo y Moraga han colaborado como ponentes en conferencias o investigadores en estudios patrocinados por alguno de los siguientes: Wyeth/Pfizer, Sanofi-Pasteur-MSD, GlaxoSmithKline, Novartis, Crucell, Esteve, Abbvie y Astra-Zéneca. El resto de los autores declara no tener conflicto de intereses.
ABREVIATURAS: ORL: Otorrinolaringología • RM: resonancia nuclear magnética • TC: tomografía computarizada.
BIBLIOGRAFÍA
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;50(Supl 23):1-298.
- American Academy of Pediatrics. Subcommittee on Management of Sinusitis and Committee on Quality Improvement. Clinical practice guideline: management of sinusitis. Pediatrics. 2001;108(3):798-808. Erratum in: Pediatrics. 2002;109(5):40. Pediatrics. 2001;108(5):A24.
- Meltzer EO, Hamilos DL. Rhinosinusitis diagnosis and management for the clinician: a synopsis of recent consensus guidelines. Mayo Clin Proc. 2011;86(5):427-43.
- McQuillan L, Crane LA, Kempe A. Diagnosis and management of acute sinusitis by pediatricians. Pediatrics. 2009;123(2):e193-8.
- Tomás M, Ortega P, Mensa J, García J, Barberán J. Diagnóstico y tratamiento de las rinosinusitis agudas. Segundo consenso. Rev Esp Quimioter. 2008;21(1):45-59.
- Jones N. The nose and paranasal sinuses physiology and anatomy. Adv Drug Deliv Rev. 2001;51(1-3):5-19.
- Gwaltney JM. Clinical significance and pathogenesis of viral respiratory infections. Am J Med. 2002;112 Suppl 6A:13S-18S.
- Marchisio P, Ghisalberti E, Fusi M, Baggi E, Ragazzi M, Dusi E. Paranasal sinuses and middle ear nfections: what do they have in common? Pediatr Allergy Immunol. 2007;18 Suppl 18:31-4.
- Beule AG. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. Laryngorhinootologie. 2010;89 Suppl 1:S15-34.
- Goldsmith AJ, Rosenfeld RM. Treatment of pediatric sinusitis. Pediatr Clin North Am. 2003;50(2):413-26.
- Gordts F, Halewyck S, Pierard D, Kaufman L, Clement PA. Microbiology of the middle meatus: a comparison between normal adults and children. J Laryngol Otol. 2000;114(3):184-8.
- Gordts F, Abu Nasser I, Clement PA, Pierard D, Kaufman L. Bacteriology of the middle meatus in children. Int J Pediatr Otorhinolaryngol. 1999;48(2):163-7.
- Brook I. Management of Bacterial Rhinosinusitis in Children. Eur Respir Dis. 2012;8(1):56-60.
- Brook I. Microbiology of sinusitis. Proc Am Thorac Soc. 2011;8(1):90-100.
- Brook I. Bacteriology of acute and chronic ethmoid sinusitis. J Clin Microbiol. 2005;43(7):3479-80.
- Wald ER. Microbiology of acute and chronic sinusitis in children and adults. Am J Med Sci. 1998;316(1):13-20.
- Brook I. Discrepancies in the recovery of bacteria from multiple sinuses in acute and chronic sinusitis. J Med Microbiol. 2004;53(Pt 9):879-85.
- Brook I, Gober AE. Frequency of recovery of pathogens from the nasopharynx of children with acute maxillary sinusitis before and after the introduction of vaccination with the 7-valent pneumococcal vaccine. Int J Pediatr Otorhinolaryngol. 2007;71(4):575-9.
- Brook I, Foote PA, Hausfeld JN. Frequency of recovery of pathogens Rausing acute maxillary sinusitis in adults before and after introduction of vaccination of children with the 7-valent pneumococcal vaccine. J Med Microbiol. 2006;55(Pt 7):943-6.
- Moreno-Pérez D, Álvarez García FJ, Arístegui Fernández J, Barrio Corrales F, Cilleruelo Ortega MJ, Corretger Rauet JM, et al.; Comité Asesor de Vacunas de la Asociación Española de Pediatría, España. Calendario de vacunaciones de la AEP: recomendaciones 2013. An Pediatr (Barc). 2013;78(1):59.e1-27.
- Liñares J, Ardanuy C, Pallares R, Fenoll A. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin Microbiol Infect. 2010;16(5):402-10.
- Fenoll A, Granizo JJ, Aguilar L, Giménez MJ, Aragoneses-Fenoll L, Hanquet G, et al. Temporal trends of invasive Streptococcus pneumoniae serotypes and antimicrobial resistance patterns in Spain from 1979 to 2007. J Clin Microbiol. 2009;47(4):1012-20.
- Sánchez-Tatay D, Arroyo LA, Tarragó D, Lirola MJ, Porras A, Fenoll A, et al. Antibiotic susceptibility and molecular epidemiology of nasopharyngeal pneumococci from Spanish children. Clin Microbiol Infect. 2008;14(8):797-801.
- Fenoll A, Aguilar L, Vicioso MD, Giménez MJ, Robledo O, Granizo JJ. Increase in serotype 19A prevalence and amoxicillin non-susceptibility among paediatric Streptococcus pneumoniae isolates from middle ear fluid in a passive laboratory-based surveillance in Spain, 1997-2009. BMC Infect Dis. 2011;11:239.
- Picazo JJ. Management of antibiotic-resistant Streptococcus pneumoniae infections and the use of pneumococcal conjugate vaccines. Clin Microbiol Infect. 2009;15 Suppl 3:4-6.
- García-Cobos S, Campos J, Cercenado E, Román F, Lázaro E, Pérez-Vázquez M, et al. Antibiotic resistance in Haemophilus influenzae decreased, except for beta-lactamase-negative amoxicillin-resistant isolates, in parallelwith community antibiotic consumption in Spain from 1997 to 2007. Antimicrob Agents Chemother. 2008;52(8):2760-6.
- Aracil B, Gómez-Garcés JL, Alós JI; Grupo de Estudio de Infección en Atención Primaria de la SEIMC (IAP-SEIMC). Sensibilidad de Haemophilus influenzae aislados en España a 17 antimicrobianos de administración oral. Enferm Infecc Microbiol Clin. 2003;21(3):131-6.
- Oteo J, Campos J. Valor de los sistemas de vigilancia de resistencia a antibióticos. Enferm Infecc Microbiol Clin. 2003;21(3):123-5.
- Wald E, Kaplan S, Friedman E, Wood R. Acute bacterial rhinosinusitis in children: Clinical features and diagnosis. UpToDate (update 12/6/2012) [en línea] [consultado el 12/nov/2012]. Disponible en www.uptodate.com/contents/acute-bacterial-rhinosinusitis-in-children-clinical-features-and-diagnosis
- Pappas E, Hendley J. Sinusitis. En: Kliegman R, Behrman R, Jenson H, Stanton B. Nelson Tratado de Pediatría, 18.ª edición española. Barcelona: Elsevier; 2009. p. 1749-52.
- Mori F, Fiocchi A, Barni S, Beghi G, Caddeo A, Calcinai E, et al. Management of acute rhinosinusitis. Pediatr Allergy Immunol. 2012;23 Suppl s22:27-31.
- DeMuri GP, Wald ER. Complications of acute bacterial sinusitis in children. Pediatr Infect Dis J. 2011;30(8):701-2.
- Díez O, Batista N, Bordes A, Lecuona M, Lara M. Diagnóstico microbiológico de las infecciones del tracto respiratorio superior. Enferm Infecc Microbiol Clin. 2007;25(6):387-93.
- Chow AW, Benninger MS, Brook I, Brozek JL, Goldstein EJ, Hicks LA, et al. Infectious Diseases Society of America. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54(8):e72-e112.
- Pappas DE, Hendley JO, Hayden FG, Winther B. Symptom profile of common colds in school-aged children. Pediatr Infect Dis J. 2008;27(1):8-11.
- DeMuri, Wald E. Acute Sinusitis: Clinical Manifestations and Treatment Approaches. Pediat Ann. 2010;39:34-40.
- Manning SC, Biavati MJ, Phillips DL. Correlation of clinical sinusitis signs and symptoms to imaging findings in pediatric patients. Int J Pediatr Otorhinolaryngol. 1996;37(1):65-74.
- Glasier CM, Mallory GB Jr, Steele RW. Significance of opacification of the maxillary and ethmoid sinuses in infants. J Pediatr. 1989;114(1):45-50.
- American college of radiology ACR Aproppiateness criteria. 2012 [en línea] [consultado el 26/abr/2013]. Disponible en www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/SinusitisChild.pdf
- von Kalle T, Fabig-Moritz C, Heumann H, Winkler P. Incidental findings in paranasal sinuses and mastoid cells: a cross-sectional magnetic resonance imaging (MRI) study in a pediatric radiology department. Rofo. 2012;184(7):629-34.
- McAlister WH. Imaging of sinusitis in infants and children. In: Lusk RP (ed.). Pediatric Sinusitis. New York, NY: Raven Press; 1992. p. 15-42.
- Castellanos J, Axelrod D. Flexible fiberoptic rhinoscopy in the diagnosis of sinusitis. J Allergy Clin Immunol. 1989;83(1):91-4.
- Otten FW, Grote JJ. The diagnostic value of transillumination for maxillary sinusitis in children. Int J Pediatr Otorhinolaryngol. 1989;18(1):9-11.
- Karantanas AH, Sandris V. Maxillary sinus inflammatory disease: ultrasound compared to computed tomography. Comput Med Imaging Graph. 1997;21(4):233-41.
- Tiedjen KU, Becker E, Heimann KD, Knorz S, Hildmann H. Value of B-image ultrasound in diagnosis of paranasal sinus diseases in comparison with computerized tomography. Laryngorhinootologie. 1998;77(10):541-6.
- de La Flor J, Parellada N. Correlació entre simptomatologia clínica sospitosa de sinusitis i presencia d’hipertròfia de mucosa i/o exsudat de sins maxillars, i d’exsudat de sins frontals, detectats amb ultrasonografia portátil en una consulta de Pediatría d’atenció primària. Pediatr Catalana. 2005;63:65-76.
- Acute Bacterial Sinusitis Guideline Team, Acute Bacterial Sinusitis Guideline Team, Cincinnatti Children’s Hospital MedicalCenter: Evidence-Based Care Guideline for medical management of Acute Bacterial Sinusitis in children 1 through 17 yearsof age [en línea]. Disponible en www.cincinnatichildrens.org/workarea/linkit.aspx?linkidentifier=id&itemid=87964&libid=87652
- Chang AB, Glomb WB. Guidelines for evaluating chronic cough in pediatrics: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(Suppl 1):260s-283s.
- Clinical Knowledge Summaries. NICE. Sinusitis. Additional information [en línea] [consultado el 30/mar/2013]. Disponible en http://cks.nice.org.uk/sinusitis#!diagnosisadditional
- Kristo A, Uhari M. Timing of rhinosinusitis complications in children. Pediatr Infect Dis J. 2009;28(9):769-71.
- Sultész M, Csákányi Z, Majoros T, Farkas Z, Katona G. Acute bacterial rhinosinusitis and its complications in our pediatric otolaryngological department between 1997 and 2006. Int J Pediatr Otorhinolaryngol. 2009;73(11):1507-12.
- Rumelt S, Rubin PA. Potential sources for orbital cellulitis. Int Ophthalmol Clin. 1996;36(3):207-21.
- Chandler JR, Langenbrunner DJ, Stevens ER. The pathogenesis of orbital complications in acute sinusitis. Laryngoscope. 1970;80(9):1414-28.
- Georgakopoulos CD, Eliopoulou MI, Stasinos S, Exarchou A, Pharmakakis N, Varvarigou A. Periorbital and orbital cellulitis: a 10-year review of hospitalized children. Eur J Ophthalmol. 2010;20(6):1066-72.
- Sobol SE, Marchand J, Tewfik TL, Manoukian JJ, Schloss MD. Orbital complications of sinusitis in children. J Otolaryngol. 2002;31(3):131-6.
- Bayonne E, Kania R, Tran P, Huy B, Herman P. Intracranial complications of rhinosinusitis. A review, typical imaging data and algorithm of management. Rhinology. 2009;47(1):59-65.
- Coenraad S, Buwalda J. Surgical or medical management of subperiosteal orbital abscess in children: a critical appraisal of the literature. Rhinology. 2009;47(1):18-23.
- Jones NS, Walker JL, Bassi S, Jones T, Punt J. The intracranial complications of rhinosinusitis: can they be prevented? Laryngoscope. 2002;112(1):59-63.
- Gavriel H, Yeheskeli E, Aviram E, Yehoshua L, Eviatar E. Dimension of subperiosteal orbital abscess as an indication for surgical management in children. Otolaryngol Head Neck Surg. 2011;145(5):823-7.
- Siedek V, Kremer A, Betz CS, Tschiesner U, Berghaus A, Leunig A. Management of orbital complications due to rhinosinusitis. Eur Arch Otorhinolaryngol. 2010;267(12):1881-6.
- Hoxworth JM, Glastonbury CM. Orbital and intracranial complications of acute sinusitis. Neuroimaging Clin N Am. 2010;20(4):511-26.
- Gunn VL, Taha SH, Liebelt EL, Serwint JR. Toxicity of over-the-counter cough and cold medications. Pediatrics. 2001;108(3):E52.
- Shaikh N, Wald ER, Pi M. Decongestants, antihistamines and nasal irrigation for acute sinusitis in children. Cochrane Database Syst Rev. 2012 Sep 12;9:CD007909.
- Hauptman G, Ryan MW. The effect of saline solutions on nasal patency and mucociliary clearance in rhinosinusitis patients. Otolaryngol Head Neck Surg. 2007;137(5):815-21.
- Wang YH, Yang CP, Ku MS, Sun HL, Lue KH. Efficacy of nasal irrigation in the treatment of acute sinusitis in children. Int J Pediatr Otorhinolaryngol. 2009;73(12):1696-701.
- Wang YH, Ku MS, Sun HL, Lue KH. Efficacy of nasal irrigation in the treatment of acute sinusitis in atopic children. J Microbiol Immunol Infect. 2012 Sep 30. pii: S1684-1182(12)00179-X.
- Kassel JC, King D, Spurling GK. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database Syst Rev. 2010 Mar17;(3):CD00682.
- Venekamp RP, Thompson MJ, Hayward G, Heneghan CJ, Del Mar CB, Perera R, et al. Systemic corticosteroids for acute sinusitis. Cochrane Database Syst Rev. 2011 Dec 7;(12):CD008115.
- Zalmanovici A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2011 May 8(4):CD005149.
- Sinclair CF, Berkowitz RG. Prior antibiotic therapy for acute sinusitis in children and the development of subperiosteal orbital abscess. Int J Pediatr Otorhinolaryngol. 2007;71(7):1003-6.
- Falagas ME, Giannopoulou KP, Vardakas KZ, Dimopoulos G, Karageorgopoulos DE. Comparison of antibiotics with placebo for treatment of acute sinusitis: a meta-analysis of randomised controlled trials. Lancet Infect Dis. 2008;8(9):543-52.
- Blin P, Blazejewski S, Lignot S, Lassalle R, Bernard MA, Jayles D, et al. Effectiveness of antibiotics for acute sinusitis in real-life medical practice. Br J Clin Pharmacol. 2010;70(3):418-28.
- Guarch Ibáñez B, Buñuel Álvarez JC, López Bermejo A, Mayol Canalsa L. El papel de la antibioterapia en la sinusitis aguda: revisión sistemática y metaanálisis. An Pediatr (Barc). 2011;74:154-60.
- Wald ER. Treatment of acute sinusitis. Pediatr Infect Dis J. 2010;29(1):94.
- Hansen FS, Hoffmans R, Georgalas C, Fokkens WJ. Complications of acute rhinosinusitis in The Netherlands. Fam Pract. 2012;29(2):147-53.
- Méndez Hernández M, Rodrigo Gonzalo de Liria C. Sinusitis aguda. Celulitis periorbitaria. Protocolos de Infectología de la Asociación Española de Pediatría y la Sociedad Española de Infectología Pediátrica. Año 2011 [en línea] [consultado el 15/oct/2012]. Disponible en www.aeped.es/sites/default/files/documentos/sinusitis.pdf
- American Academy of Pediatrics. Principles of appropriate use for upper respiratory tract infections. En: Pickering LK (ed.). Red Book. 2012. Report of the Committee on Infectious Diseases, 29th, Elk Grove Village, IL: American Academy of Pediatrics; 2012. p.802.
- Del Castillo Martín F, Baquero Artigao F, de la Calle Cabrera T, López Robles MV, Ruiz Canela J, Alfayate Miguelez S, et al. Documento de consenso sobre etiología, diagnóstico y tratamiento de la otitis media aguda. Rev Pediatr Aten Primaria. 2012;14:195-205 [en línea] [consultado el 16/ago/2013].
- Kristo A, Uhari M, Luotonen J, Ilkko E, Koivunen P, Alho OP. Cefuroxime axetil versus placebo for children with acute respiratory infection and imaging evidence of sinusitis: a randomized, controlled trial. Acta Paediatr. 2005;94(9):1208-13.
- Karageorgopoulos DE, Giannopoulou KP, Grammatikos AP, Dimopoulos G, Falagas ME. Fluoroquinolones compared with beta-lactam antibiotics for the treatment of acute bacterial sinusitis: a meta-analysis of randomized controlled trials. CMAJ. 2008;178(7):845-54.
- Institute for Clinical Systems Improvement (ICSI). Diagnosis and treatment of respiratory illness in children and adults. Third Edition. January 2011[en línea] [consultado el 15/oct/2012]. Disponible en www.icsi.org
- Wald ER, Kaplan SL, Isaacson GC, Wood RA, Torchia MM. Acute bacterial rhinosinusitis in children: Microbiology and treatment. UpToDate (updated 18/09/12) [en línea] [consultado el 18/oct/2012]. Disponible en www.uptodate.com/home/





