Vol. 14 - Num. 53
Originales
Vacunas infantiles no financiadas, ¿cuál es la cobertura en un centro de salud urbano?
Marta Esther Vázquez Fernándeza, P Bustamante Marcosb, B Herrero Bregónc, MF Muñoz Morenod, M de Santiago Garcíae, L Barriada Álvarezf
aPediatra. CS Arturo Eyries. Facultad de Medicina. Universidad de Valladolid. Valladolid. España.
bMIR-MFyC. CS Arturo Eyries. Valladolid. España.
cMIR-MFyC. CS Arturo Eyries. (Área Oeste). Valladolid. España.
dUnidad de Apoyo a la Investigación. Hospital Clínico Universitario. Valladolid. España.
ePediatra. CS Arturo Eyries. Área Oeste Valladolid. Valladolid. España.
fEnfermera. CS Arturo Eyries. Área Oeste Valladolid. Valladolid. España.
Correspondencia: ME Vázquez. Correo electrónico: mvmarvazfer@gmail.com
Cómo citar este artículo: Vázquez Fernández ME, Bustamante Marcos P, Herrero Bregón B, Muñoz Moreno MF, de Santiago García M, Barriada Álvarez L. Vacunas infantiles no financiadas, ¿cuál es la cobertura en un centro de salud urbano? Rev Pediatr Aten Primaria. 2012;14:21-6.
Publicado en Internet: 22-03-2012 - Número de visitas: 21897
Resumen
Objetivo: determinar la tasa de cobertura de las vacunas infantiles no financiadas y si son administradas correctamente, en los niños atendidos recientemente en un centro de salud urbano de nivel socioeconómico medio.
Material y métodos: se realiza un estudio transversal, descriptivo, analizando el registro de vacunas frente al neumococo, rotavirus y varicela de la historia informatizada de los niños atendidos por dos pediatras, que hayan podido recibir todas las dosis recomendadas.
Resultados: se han incluido 162 niños susceptibles de vacunación frente a neumococo, 112 para el rotavirus y 160 para varicela. Las tasas de cobertura para la vacunación completa según recomendaciones del Comité Asesor de Vacunas han sido el 64,81, el 66 y el 58,1%, respectivamente. El 11,73% de los que inician la vacunación frente al neumococo y el 2,68% del rotavirus no completan el número de dosis recomendadas. No hubo diferencias de vacunación significativas entre ambos pediatras.
Conclusiones: nos encontramos con unos niveles de cobertura vacunal medios y con pautas incompletas. Los recursos económicos de las familias y la labor educativa del personal de Enfermería y Pediatría son los responsables de estas tasas vacunales. Desconocemos el efecto epidemiológico sobre las enfermedades que se quieren prevenir, de estas tasas vacunales. Es necesario fomentar la equidad en las prestaciones sanitarias relacionadas con las vacunas.
Palabras clave
● Cobertura de vacunación ● Rotavirus ● Streptococcus pneumoniae ● Vacunación ● VaricelaINTRODUCCIÓN
En la actualidad, existen varias vacunas infantiles que no se incluyen en el calendario de vacunación oficial de la mayoría de las comunidades autónomas1. Corresponde a los padres decidir y costear si sus hijos son inmunizados contra otras enfermedades infecciosas2.
En esta situación, los pediatras de Atención Primaria, como agentes de la salud pública de la comunidad, como abogados personales del niño y siguiendo las recomendaciones del Comité Asesor de Vacunas de la Asociación Española de Pediatría, consideran necesario que los padres de cada niño tengan derecho a conocer y decidir las medidas preventivas que pueden beneficiar a su hijo. De esta manera, se ofrece sistemáticamente de forma anticipatoria, en las revisiones de salud, información oral y por escrito de las vacunas no financiadas por el sistema de salud.
A pesar de ello, la cobertura vacunal de este grupo de vacunas es pocas veces estimada directamente de nuestras consultas. El objetivo principal de este trabajo ha sido determinar, en los niños atendidos más recientemente en nuestro centro de salud, la tasa de vacunación y si se cumplen los intervalos y el número de dosis recomendados, de las tres vacunas no financiadas por el Sistema Nacional de Salud de nuestra comunidad autónoma (Castilla y León): vacuna conjugada del neumococo, vacuna del rotavirus y vacuna de la varicela.
MATERIALES Y MÉTODOS
Hemos realizado un estudio descriptivo, transversal y retrospectivo a partir de los datos vacunales recogidos en la historia clínica informatizada del programa MEDORA. La fecha de cierre en la recogida de datos ha sido el 30 de diciembre de 2011. Como población diana, se recogieron los registros más recientes existentes de vacunación infantil de los niños que hayan podido completar todas las dosis, en un centro de salud urbano de nivel socioeconómico medio, el Centro de Salud Arturo Eyries, atendido por dos pediatras y una enfermera, con un promedio de 2350 niños entre los dos cupos. Se excluyeron del estudio los niños con escaso o ningún registro clínico, los que habían sido baja por traslado de centro de salud o fuera de la capital y aquellos que habían sido alta en nuestro centro de salud durante los periodos de análisis.
Para la vacuna del neumococo se consideró el número de dosis administradas y la edad de inicio. Se establecieron así los siguientes grupos de análisis: menores de seis meses que recibían cuatro dosis; niños entre los seis meses y el año, con tres dosis y mayores de un año que se ponían dos dosis. En todos los casos, la última dosis debía ser administrada antes de los dos años. Por tanto, quedaron incluidos en el estudio los niños mayores de dos años nacidos entre el 1 de enero de 2009 y el 1 de enero de 2010; en total, 162 niños.
El grupo de estudio para la vacuna del rotavirus fueron los niños mayores de seis meses, nacidos entre el 1 de septiembre de 2009 y el 1 de junio de 2010, en total 112 niños. Solo se analizaron nueve meses porque la vacunación contra el rotavirus estuvo suspendida de junio a diciembre de 2010, por un problema de calidad relacionado con la posible contaminación por fragmentos de circovirus porcino. Se consideró bien vacunado a quien hubiera recibido tres dosis antes de las 26 semanas de edad, tal y como se recoge en la ficha técnica de la vacuna (en el momento del estudio) disponible en las farmacias, Rotateq®.
Teniendo en cuenta que se recomienda la administración de la vacuna de la varicela entre los 12 y los 15 meses, los datos referentes a la misma corresponden a la cohorte de niños mayores de 16 meses (un mes de calendario más como límite de tiempo para considerarlo bien vacunado), nacidos durante el periodo del 1 de septiembre de 2009 al 1 de septiembre de 2010, en total 160 niños. No pudimos valorar la revacunación, porque la edad a la que se aconseja esta segunda dosis, los tres o cuatro años, los niños de nuestro estudio no han podido completar la pauta de dos dosis vacunales que se recomienda desde el año 20081.
Por ultimo, se analizó, mediante la Chi cuadrado de Pearson y el programa Epidat® versión 3.1, si existía influencia del pediatra que informaba en el estado de vacunación.
RESULTADOS
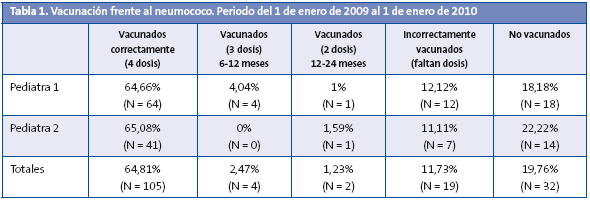
Destacamos que durante el periodo de análisis de la vacunación antineumocócica observamos la coexistencia de tres tipos de vacunas conjugadas: heptavalente (Prevenar®), decavalente (Synflorix®) y 13-valente (Prevenar13®). Con independencia de la vacuna administrada, del total de población susceptible (162 niños) el 80,25% (n = 130) inició la administración de alguna dosis y el 19,76% (n = 32) no ha recibido ninguna dosis. Del total de niños que han iniciado la vacunación, el 11,73% (n = 19) estaba mal vacunado porque les faltaban dosis, el 2,47% (n = 4) había recibido tres dosis, iniciando su primera dosis entre los 6 y los 12 meses, y el 1,23% (n = 2), dos dosis entre el primer y el segundo año de vida. Tan solo el 64,81% del total de la población susceptible ha recibido cuatro dosis: la primera dosis se pone a los dos meses de vida; la segunda, a los cuatro o cinco; la tercera, a los seis o siete, y la cuarta, entre 12 y 24 meses, generalmente a los 18 meses. Los resultados del análisis se muestran en la Tabla 1.
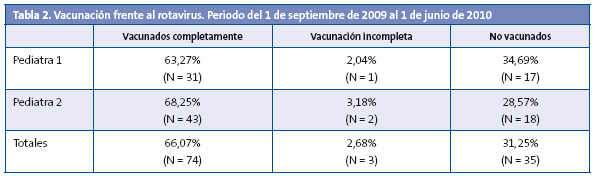
Respecto a la vacunación frente al rotavirus, los resultados que se reflejan en la Tabla 2 son los siguientes: el 66% de los niños con edad para vacunación ha recibido correctamente las tres dosis vacunales antes de los seis meses, el 31,25% no ha recibido ninguna dosis, y por tanto no está vacunado, y al 2,68% le ha faltado alguna dosis, y por tanto está mal vacunado.
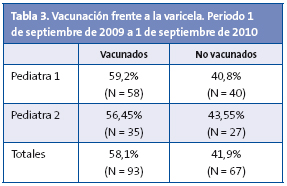
Por último, la vacuna frente a la aricela mostró porcentajes del 58,1% de niños vacunados frente al 41,9% de no vacunados (Tabla 3).
No encontramos diferencias significativas de vacunación en función del pediatra que atendía al niño e informaba a los padres: P = 0,557 para la vacuna del neumococo, P = 0,753 para la vacuna del rotavirus y P = 0,732 para la de la varicela. Ni tampoco en los que recibían vacunación incompleta del neumococo (P = 0,409) y del rotavirus (P = 0,582).
DISCUSIÓN
Nuestro estudio, aunque muy local, ofrece cifras muy reales de coberturas para las tres vacunas infantiles no incluidas en el calendario oficial. Los resultados obtenidos reflejan unas tasas de vacunación similares para la vacuna del neumococo y del rotavirus, y una tasa más baja para la vacuna de la varicela, quizá debido a la percepción de que esta enfermedad es un proceso más banal y a que en nuestra comunidad existe la posibilidad de vacunación a los 11 años, si aún no han padecido la enfermedad.
Estas cifras las consideramos bajas, si tenemos en cuenta que las coberturas de las vacunas incluidas en el calendario vacunal oficial, según datos del Ministerio de Sanidad, superan el 95% tanto para la primovacunación como para las dosis de refuerzo3 y que para generar una buena inmunidad de grupo lo aconsejable es conseguir tasas de vacunación mayores del 70-80%4.
Aunque se pueden considerar elevadas, dadas las características de “vacunas no financiadas ni incluidas en el calendario vacunal” y los datos de cobertura vacunal obtenidos por otros autores a nivel local5-7, en España, en 2003, la cobertura de vacunación antineumocócica infantil, estimada a través del número de dosis vendidas en las farmacias españolas, rondaba el 50%, variando en las distintas comunidades autónomas8. Esto se debe a la labor de educación sanitaria realizada por parte del personal de Enfermería y Pediatría, que va recordando a los padres en cada revisión la fecha de la próxima vacuna. Como era de esperar, no encontramos diferencias significativas en la cobertura vacunal de los niños atendidos por los dos pediatras, ya que atienden una población de nivel socioeconómico similar y realizan el mismo tipo de consejo oral y por escrito en las revisiones de salud.
Parece que los mayores limitantes para la administración de las vacunas son los precios elevados. La vacuna antineumocócica (cuatro dosis) cuesta aproximadamente 300 euros, la del rotavirus (tres dosis) 200 euros y la de la varicela (dos dosis) alrededor de 120 euros. También la percepción por parte de los padres de un número elevado de inyecciones en lactantes.
Destacamos como hallazgo significativo un porcentaje considerable de niños que no recibe el total de cuatro dosis recomendadas frente al neumococo (15,43%) y menor frente al rotavirus (2,68%). En el momento actual, no existen datos de seguridad y eficacia para ambas vacunas cuando se administran en pautas incompletas, ni siquiera para las pautas “alternativas” de vacunación a niños mayores con un menor número de dosis, tanto para la prevención de enfermedad como sobre la inmunidad colectiva10. La causa no ha podido establecerse con claridad: olvido, problemas económicos o razonamiento erróneo sobre su eficacia.
Desconocemos el efecto epidemiológico de estas tasas medias de cobertura vacunal y de las vacunaciones incompletas, sobre las enfermedades que se quieren prevenir9. Preocupa principalmente el desplazamiento de casos a edades mayores, así como la emergencia de serotipos no vacunales, con mayor gravedad o complicaciones, relacionados con la desigual inmunidad de grupo y la disminución la de circulación de los agentes infecciosos incluidos en las vacunas10-13.
La inclusión de estas vacunas en los calendarios vacunales oficiales de las comunidades autónomas en España14 y en otros países europeos15 y americanos es variable en la actualidad16:
- La vacuna antineumocócica solo está incluida en el calendario vacunal madrileño, desde el año 2006. También en estados norteamericanos y europeos de nuestro entorno.
- La vacuna del rotavirus no entra en el calendario vacunal de ninguna comunidad autónoma española. En América se administra de forma sistemática, y en Europa solo en algunos países, como Austria.
- En Castilla y León, la vacuna de la varicela está incluida desde el año 2005 en el calendario de vacunaciones infantiles para los niños de 11 años que no hubieran pasado aún la enfermedad. Sin embargo, la Asociación Española de Pediatría aconseja la vacunación de todos los niños entre los 12 y los 15 meses, y la revacunación entre los tres y los cuatro años17. Madrid y Navarra son las únicas comunidades autónomas en las que la vacuna de la varicela está incluida en su calendario oficial, en el segundo año de vida. En EE. UU. y en distintas provincias de Canadá está financiada desde hace varios años. En Europa se está implantando la vacunación universal, como en Alemania, Grecia e Italia.
Conclusiones
Conseguimos tasas de cobertura vacunal inadecuadas para lograr una inmunidad de grupo completa, con un porcentaje importante de niños incorrectamente vacunados.
Consideramos que el elevado precio ha podido constituir el principal elemento disuasorio para la no vacunación.
La variabilidad en la administración de estas vacunas debe tenerse en cuenta al valorar el efecto epidemiológico sobre estas enfermedades. Sería necesario un sistema de control a nivel nacional de las tasas de cobertura de estas vacunas no financiadas.
Proponemos más estudios de coste directo e indirecto que aclaren la necesidad de su inclusión en los calendarios oficiales de vacunación infantil, a fin de que exista equidad entre personas con recursos económicos variables.
BIBLIOGRAFÍA
- Moreno-Pérez D, Álvarez García FJ, Arístegui Fernández J, Barrio Corrales F, Cilleruelo Ortega MJ, Corretger Rauet JM et al. Calendario de vacunaciones de la Asociación Española de Pediatría: recomendaciones 2012. An Pediatr (Barc). 2012;76:42.e1-e23.
- Merino Moína M. Vacunas infantiles ¿cuál es nuestro papel como pediatras de Atención Primaria [consultado el 23/01/2012] Disponible en www.spapex.es/vacunas.htm
- Cobertura de vacunas. Datos estadísticos, total nacional 2002-2010. Ministerio de Sanidad, Políticas Sociales e Igualdad [consultado el 23/01/2012]. Disponible en www.msc.es/profesionales/saludPublica/prevPromocion/vacunaciones/coberturas.htm
- Ministerio de Sanidad, Políticas Sociales e Igualdad. Varicela. Recomendaciones de vacunación y sus implicaciones en salud pública, 2005 [consultado el 23/01/2012]. Disponible en www.msc.es
- Ramos Salas E, Díez Delgado FJ, Salazar Agulló M, Ramos Pleguezuelos FM. Coberturas de vacunación neumocócica en menores de 2 años en 2 zonas de Almería capital. Vacunas. 2008;9:12-8.
- Vila A, de Diego C, Salchench E, Saun N. Coberturas de vacunación antineumocócica con vacuna heptavalente conjugada en la población infantil de Tarragona-Valls. Aten Primaria. 2007;39:507.
- Hernández Pascual M, Ruiz Serrano A, Rodríguez Ortiz de Salazar MI, Casado López M, López de Andrés A. Cobertura vacunal frente al rotavirus en la población infantil del Área 8 de la Comunidad de Madrid. Vacunas. 2008;9:117-20.
- Comité Asesor de Vacunas de la AEP. Comentarios del Comité Asesor de Vacunas (CAV) de la Asociación Española de Pediatría (AEP) al documento emitido por el Ministerio de Sanidad (DM) español en abril de 2006 “Enfermedad Invasora por Streptococcus pneumoniae. Implicaciones de la vacunación con vacuna conjugada heptavalente” [consultado el 23/01/2012]. Disponible en http://vacunasaep.org/documentos/comentarios-del-cav-al-documento-sobre-neumococo-del-ministerio-de-sanidad (actualizado el 05-04-2006).
- American Academy of Pediatrics. Active and passive immunization. En: Pickering LK, Baker CJ, Kimberlin DW, Long SS (eds.). 2009 Red Book: report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2009.
- Singleton RJ, Hennessy TW, Bulkow LR. Invasive pneumococcal disease caused by non vaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297:1784-92.
- Black S, Shinefield H, Baxter R. Impact of the use of heptavalent pneumococcal conjugate vaccine on disease epidemiology in children and adults. Vaccine. 2006;24 (suppl 2):S2-S80.
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11-22.
- Bayer O, Heininger U, Heiligensetzer C, Von Kries R. Metaanalisis of vaccine effectiveness in varicella outbreaks. Vaccine. 2007;25:6655-60.
- Bernaola Iturbe E, Giménez Sánchez F, Baca Cots M, de Juan Martín F, Diez Domingo J, Garcés Sánchez M. Recomendaciones de vacunación de la Asociación Española de Pediatría 2008. Vacunas. 2008;9:80-5.
- Asociación Española de Pediatría de Atención Primaria. Calendarios de vacunación españoles y extranjeros [consultado el 23/01/2012]. Disponible en www.aepap.org/vacunas/calendarios-espanoles/calendarios-extranjeros
- Advisory Committee on Immunization Practices (ACIP). CDC. Recommended immunization schedules for persons aged 0-18 years. Unites States 201. MMWR. 2011;60;1-4.
- Ministerio de Sanidad y Consumo. Dirección General de Salud Pública. Varicela: epidemiología y situación actual. Vacunas: características y eficacia/efectividad. Recomendaciones de vacunación y sus implicaciones en Salud Pública. 2005 [consultado el 23/01/2012].





