Vol. 26 - Num. 102
Original Papers
Evaluation of effectiveness of education in the management of epinephrine autoinjectors
María Villarreal Calvoa, Juan Carlos Juliá Benitob
aPediatra de Atención Primaria. Servicio Navarro de Salud. España.
bPediatra de Atención Primaria. Servicio Valenciano de Salud. España.
Reference of this article: Villarreal Calvo M, Juliá Benito JC. Evaluation of effectiveness of education in the management of epinephrine autoinjectors . Rev Pediatr Aten Primaria. 2024;26:165-71. https://doi.org/10.60147/279dc09a
Published in Internet: 14-06-2024 - Visits: 8943
Abstract
Introduction: anaphylaxis is a medical emergency and early administration of adrenaline is essential to reduce morbidity and mortality. Adrenaline autoinjectors are available for patients with a diagnosis of anaphylaxis so they can receive the medication without delay. The main goal of our study was to evaluate parental knowledge about anaphylaxis and the use of autoinjectors and whether their knowledge improved after some training.
Material and methods: pre- and post-intervention study. We analysed 6 primary care caseloads, selecting patients with a diagnosis of anaphylaxis who had adrenaline autoinjector prescriptions. Participants received 2 emails. The first one included a questionnaire about anaphylaxis and how to use the adrenaline autoinjector. The second one contained informational pages and videos and a second questionnaire. That way we compared parental knowledge before and after receiving some training.
Results: food was the main cause of anaphylaxis. Only 56.3% of the participants carried the autoinjector at all times. Comparing the knowledge before and after the intervention, we found improvement in nearly every step of the administration of adrenaline. The improvement was statistically significant when it came to the correct identification of the safety release cap (p 0.018) and the awareness of the possibility of administering a second dose (p 0.006). There was a clear improvement in the knowledge of needing to massage the injection area, but it was not statistically significant (p 0.066)
Conclusions: periodic training about how to use the autoinjectors is key to reduce morbidity and mortality in patients with anaphylaxis.
Keywords
● Allergic reaction ● Anaphylaxis ● Autoinjector device ● Education ● EpinephrineINTRODUCTION
Anaphylaxis is the most severe possible allergic reaction, with a rapid onset and potentially lethal.1,2
According to clinical practice guidelines,2 the diagnosis is based on clinical criteria and the first-line treatment intramuscular adrenaline administration. Therefore, it is essential that parents and other caregivers of children with diseases causing anaphylaxis (other relatives, teachers, etc) know which allergen triggers the reaction, the characteristic symptoms and how to manage the medication so that they can deliver treatment correctly and effectively.
Anaphylaxis is a medical emergency and delay in the administration of adrenaline increases the risk of hospitalization and death due to anaphylaxis.2 In consequence, adrenaline autoinjectors are available for patients with a previous diagnosis of anaphylaxis, enabling them to initiate treatment without having to wait for a health care professional.
The aim of our study was to assess the knowledge of parents of children with anaphylaxis (general notions about the disease and how to use adrenaline autoinjectors) and whether this knowledge improved after providing a brief specific training on the subject. The ultimate goal was to improve the knowledge and skills of caregivers of affected children to reduce the morbidity and mortality associated with anaphylaxis.
MATERIAL AND METHODS
Study design and sample selection
We conducted a pre- and post-intervention comparative study in patients with a diagnosis of anaphylaxis and a prescription for intramuscular auto-injectable adrenaline.
Inclusion criteria: we included all children with a documented diagnosis of anaphylaxis in the health record who also had a prescription for adrenaline autoinjectors. We recruited patients over a period of approximately 5 months.
Exclusion criteria: patients who had not been diagnosed prior to enrolment in the anaphylaxis study and those in whom anaphylaxis had been suspected initially but in whom further evaluation of allergy had ruled it out, who therefore did not have a prescription for an adrenaline autoinjector.
Sample selection: we identified candidates in 6 primary care caseloads of approximately 1000-1100 children each. Through a search of the electronic health record system, we found that each caseload included 3 to 6 children who met the inclusion criteria. We contacted the corresponding families by telephone to invite them to participate in the study, explaining its characteristics and purpose. Only those who wished to participate and provided consent received 2 mails at the email address on record.
Study protocol
Baseline (pre-intervention) study variables: in a first email, we submitted a questionnaire to collect the following information:
- Sociodemographic data: patient sex and age at diagnosis. Habitual residence setting (urban/rural) and distance by car to the nearest medical facility (in minutes).
- History of allergy: family history. Personal history of diseases related to the allergic march (atopic dermatitis /allergic rhinitis /asthma).
- Questions about allergy: type of allergy of the patient (food /insect venom/drug/respiratory). Specific trigger for the patient (e.g., hazelnut, bee venom, amoxicillin).
- Anaphylaxis-related questions: first-line treatment (answer choices: oral antihistamine /oral corticosteroid/oxygen/intramuscular adrenaline), contraindications for the use of adrenaline (yes, certain drugs/yes, several diseases /no, there are no contraindications).
- Questions about adrenaline autoinjectors: brand of owned autoinjector (Altellus/Jext/Anapen/Emerade), number of autoinjectors currently owned, habit of checking expiration date of autoinjector, whether the child carries the autoinjector at all times.
- Practical questions about how to use an autoinjector: identifying the safety release in an image, correct way to hold the autoinjector, need to remove clothes before administering the injection (true/false), site of administration of the drug, time the needle needs to stay in place, need to massage injection site after administering the medication, possibility of repeating the dose if the child continues to be unwell. Fear of using the autoinjector (yes/no), previous practice with a training autoinjector or with an expired autoinjector on an orange (yes/no).
Intervention: after submitting the first questionnaire, respondents received a second email with the intervention, which consisted in viewing educational videos and pages with infographics showing how to use adrenaline autoinjectors. All these information was validated and came from official sources (Appendix 1):
- Sociedad Española de Alergología e Inmunología clínica (SEAIC, Spanish Society of Clinical Allergology and Immunology): www.youtube.com/watch?v=g_l7ECDN-W8
- Agencia Española de Medicamentos y Productos Sanitarios (AEMPS, Spanish Agency of Medicines and Medical Devices): Altellus autoinjector (www.youtube.com/watch?v=co0v_S-Dbjw) and Yext autoinjector
- Sociedad Española de Inmunología Clínica, Alergología y Asma Pediátrica (SEICAP, Spanish Society of Paediatric Clinical Immunology, Allergology and Asthma) (Appendix 1).
Post-intervention study: the aim was to assess the impact on the knowledge about the use of autoinjectors of a brief training on the subject. With the second email, we sent access to a second survey, once again asking about the use of autoinjectors (questionnaire with the same items and same format). Last of all, we asked about the perceived usefulness of carrying continuing education activities like the one just implemented (yes/no).
As participants completed the second questionnaire, the software marked the answers in real-time. Thus, users were not able to change the answer after submitting it, but they knew whether they had answer correctly or incorrectly, learning from their mistakes.
The study adhered to ethical standards and did not violate the rights of participants. Participation in the study was voluntary and contingent on the participant providing an email address to receive the information. All responses were anonymous.
Due to the circumstances at the time of the study, in the context of the global COVID-19 pandemic, the entire study and the training intervention were carried out online.
Analysis
The statistical analysis was performed with the software package IBM SPSS Statistics (SPSS Statistics for Windows, version 22.0; IBM Corp. Armonk, NY).
We have expressed the results for qualitative variables as percentages. We summarised the results for quantitative variables as median and interquartile range. To analyse the differences in categorical variables between groups, we used the Fisher exact test (as applicable for the sample size). The level of significance was set at 5% (p < 0.05).
RESULTS
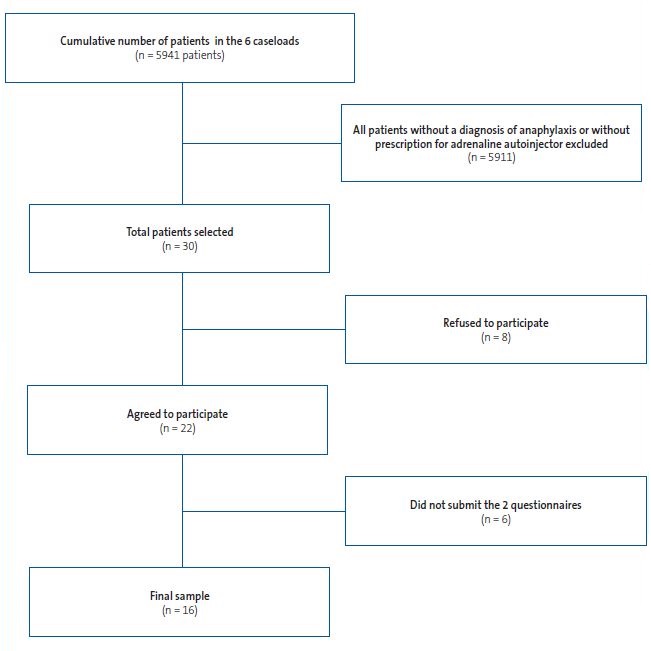
Figure 1 presents the sample selection process.
Most recruited patients lived in urban settings (81.3% vs. 18.8% in rural settings). Despite this difference, the median duration of traveling by car to a medical facility was 5 minutes (5-7).
In regard to the family history, 50% of the children did not have a family history of allergy. Of those who did, the mother was the affected individual in 18.75% of cases. In two cases (12.5%) it was the father who had an allergy. In another two, both parents had allergy, and lastly, in one case (6.25%) both parents and a sibling had a history of allergy.
Seventy-five percent of the included patients were male. More than 50% were aged 1 to 4 years, and the median age was 3.5 years (1-4.75).
All patients in the sample had received a diagnosis of an allergy march disease: atopic dermatitis in 43.75%, allergic rhinitis in 43.75% and asthma in 25% of cases.
Foods were the most frequent trigger, involved in 100% of cases. Only one patient had an additional drug allergy, but this patient was undergoing evaluation at the time of the survey and could not specify which drug triggered the symptoms. None of the patients were allergic to Hymenoptera venom.
When it came to the specific foods that triggered the allergic reaction, nearly 50% reported allergy to more than one food item, and, despite the young age of the patients, a majority were allergic to nuts (56.25%). Second in frequency was stone fruit (31.25%), followed by kiwi and legumes (18.75% in both cases), shellfish, fish and eggs (12.5%) and, lastly, cow’s milk protein (6.25%).
When asked about medication for management of anaphylaxis, it is worth noting that 100% of participants knew that the first-line treatment was intramuscular adrenaline. Furthermore, all respondents but one also knew that there are no contraindications for the administration of adrenaline.
When asked about the brand of autoinjector that had been prescribed, 56.3% had Altellus devices and 43.8% Jext devices. None of the patients had Anapen or Emerade autoinjectors. Five patients (31.3%) had 2 autoinjectors and two patients (12.5%) had 3 or more. The remaining 56.25% only had one device.
Given the importance of always carrying the medication to be able to administer it as soon as possible, it was surprising that nearly half of the children did not always carry it (56.3%).
An encouraging finding was that most respondents (87.5%) frequently checked the expiration date of the autoinjector to ensure that it was always in adequate condition should it be needed.
The items that followed concerned the use of autoinjectors, posing practical questions on the steps to perform. Our hypothesis was that the results would improve with a bit of training (Table 1).
| Table 1. Results of the pre- and post-intervention questionnaires. Comparison made with Fisher exact test | |||
|---|---|---|---|
| Item | Correct answers pre-intervention, n (%) | Correct answers post-intervention, n (%) | p |
| Identification of safety release | 10 (62.5%) | 16 (100%) | 0.018 |
| How to hold device | 13 (81.3%) | 12 (75%) | 1 |
| Need to remove clothing | 14 (87.5%) | 15 (93.8%) | 1 |
| Site of adrenaline injection | 16 (100%) | 16 (100%) | |
| Need to hold device in place a while before removing it | 13 (81.3%) | 16 (100%) | 0.226 |
| Need to massage injection site | 7 (43.8%) | 13 (81.3%) | 0.066 |
| Possibility of administering a second dose | 6 (37.5%) | 14 (87.5%) | 0.006 |

When we compared the knowledge before and after the intervention with the Fisher exact test, we found improvement after the intervention in nearly every step (Table 1). There was no uncertainty regarding the site of administration of adrenaline (in the outer thigh), which respondents knew before the intervention. The aspects that improved significantly involved the correct identification of the safety release cap (p 0.018) and the possibility of administering a second dose 5 to 15 minutes after the first one if the child continued to be unwell (p 0.006). When it came to massaging the injection site after administering the medication, there was a clear improvement in knowledge, but the difference was not statistically significant (p 0.066).
Another salient finding was that 68.8% of participants acknowledged that they were afraid to use the adrenaline autoinjector, the same percentage that reported never have practiced how to use it, either with a training autoinjector or with an expired device, on, for example, an orange.
Nearly all respondents found this activity relevant and reported that after the training they felt better prepared to act if needed. Respondents considered that this information should be provided to teachers, school canteen staff and camp counsellors for their own peace of mind and for the benefit of the child.
DISCUSSION
The increase in the prevalence of allergies,2 especially food allergies, has led to an increase in the prescribing of adrenaline autoinjectors. The European Anaphylaxis Registry reports that food items are the most frequent trigger of anaphylaxis in children (88% of reactions in children aged <6 years, 57% in children aged 6-12 years).3 The proportions in our study were higher compared to the previous literature, and food allergy was the reason for the prescription in 100% of cases. The small sample size may have affected this outcome. Nuts were the most frequent allergen in our sample, which, considering the median age of the patients (3.5 years) was consistent with the reviewed literature,2 according to which nuts (such as hazelnuts or cashews) are the most frequent trigger in early childhood. In European countries,2 stone fruits (such as peaches) are also a frequent cause of food allergy (in our case series, it was the second most frequent trigger).
There is evidence of an association between fatal anaphylaxis and the lack of adrenaline use or its incorrect administration.4 The error in administration documented most frequently in relation to the use of adrenaline autoinjectors was the unintentional injection of the medication in a finger or thumb.5,6 In our study, we analysed errors in the administration of medication, among which the most frequent were not knowing that a second dose could be administered if the patient did not improve (62.5% of errors), the need to massage the injection site (56.2% answered this incorrectly) and error in the identification of the safety release (37.5% did not identify it correctly).
The aim of our study was to assess whether these errors could be resolved with training, and we found overall improvement in autoinjector use, with significant improvement in aspects as basic as identifying the safety release cap correctly (p 0.018) or knowing that a second dose can be given 5 to 15 minutes after the first one if the child is still not well (p 0.006).
In any case, training is still useless if patients do not carry the device with them. In our series, only a little more than half the patients (56.3%) carried the device at all times. In a study published in 2019,7 patients expressed their lack of confidence in using the device correctly among the reasons not to carry the device. The authors concluded that educating patients about the importance of early treatment and prescribing devices that are easy to use along with clear instructions would increase carrying compliance. Some studies8 suggest that other factors that could be at play in not carrying the device at all times need to be considered, such as the high cost of autoinjectors, their limited shelf life, the low probability of ever using the device and fear of using it9 (an aspect observed in our study, in which nearly 70% acknowledged being afraid to use it). Some of these factors can be resolved or mitigated with education and training.
The European Medicines Agency recommends prescribing 2 autoinjectors, as approximately 10% of patients require a second dose.10 The Spanish consensus-based anaphylaxis guideline (GALAXIA 2016)1 recommends prescription of a second device under special circumstances. In our case series, more than half of the patients (56.25%) had only one device, probably in adherence to Spanish guidelines. It is important to be aware that many patients only carry one but may have another one at home, at school or in some other location, as described in previous studies.11
CONCLUSION
Continuing education and the implementation of activities designed to promote the correct use of medication (either in person or online) are key aspects in patient health education. It is very important that parents be educated in clear and understandable language about the key features of the disease and told how to avoid potential triggers of anaphylaxis.
Our study evinced that there are some very basic aspects of the use of autoinjectors that are not clear to parents and that this knowledge can improve significantly with training, increasing the confidence of parents and the safety of children.
SUPPLEMENTAL MATERIAL
Appendix 1. Uso de adrenalina autoinyectable
CONFLICTS OF INEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article. This project did not receive any funding.
AUTHORSHIP
Author contributions: original idea, supervision and editing (JCJB), survey design, patient recruitment, analysis of results and manuscript writing (MVC). The authors submitted an image release form to obtain permission to use the image in Appendix 1 (supplemental material).
REFERENCES
- Cardona V, Cabañes N, Chivato T, De la Hoz B, Fernandez M, Gangoiti I, et al. Grupo GALAXIA. Guía de Actuación en Anafilaxia. 2016. https://doi.org/10.18176/944681-8-6
- Juliá JC, Sanchez CA, Alvarado MI, Álvarez F, Arroabarren E, Capataz M, et al. Manual de anafilaxia pediátrica. SEICAP. Mayo 2017 [online] [accessed 10/06/2024]. Available at https://seicap.es/wp-content/uploads/2022/05/MAP2017-Version-2-1.pdf
- Grabenhenrich LB, Dolle S, Moneret-Vautrin A, Kohli A, Lange l, Spindler T, et al. Anaphylaxis in children and adolescents: the European anaphylaxis registry. J Allergy Clin Immunol. 2016;137(4):1128-37.e1. https://doi.org/10.1016/j.jaci.2015.11.015
- Esenboga S, Ocak M, Cetinkaya PG, Sahiner UM, Soyer O, Buyuktiryaki B, et al. Physicians prescribe adrenaline autoinjectors, do parents use them when needed? Allergol Immunopathol (Madr). 2020;48(1):3-7. https://doi.org/10.1016/j.aller.2019.07.009
- Simons FE, Lieberman PL, Read EJ Jr, Edwards ES. Hazards of unintentional injection of epinephrine from autoinjectors: a systematic review. Ann Allergy Asthma Immunol. 2009;102(4):282-7. https://doi.org/10.1016/s1081-1206(10)60332-8
- Simons FE, Edwards ES, Read EJ Jr, Clark S, Liebelt EL. Voluntarily reported unintentional injections from epinephrine auto-injectors. J Allergy Clin Immunol. 2010;125(2):419-23.e4. https://doi.org/10.1016/j.jaci.2009.10.056
- Portnoy J, Wade RL, Kessler C. Patient Carrying Time, Confidence, and Training with Epinephrine Autoinjectors: The RACE Survey. J Allergy Clin Immune Pract. 2019;7(7):2252-61. https://doi.org/10.1016/j.jaip.2019.03.021
- Murata MA, Yamamoto LG. Patient/parent administered epinephrine in acute anaphylaxis. Am J Emerg Med. 2021;46:499-502. https://doi.org/10.1016/j.ajem.2020.10.060
- Bilò MB, Martini M, Tontini C, Corsi A, Antonicelli l. Anaphylaxis Eur Ann. Allergy Clin Immunol. 2021;53(1):4-17. https://doi.org/10.23822/eurannaci.1764-1489.158
- Muraro A, Worm M, Alviani C, Cardona V, et al; European Academy of Allergy and Clinical Immunology, Food Allergy, Anaphylaxis Guidelines Group. EAACI guidelines: Anaphylaxis Allergy. 2022;77(2):357-377. https://doi.org/10.1111/all.15032
- Song TT, Brown D, Karjalainen M, Lehnigk U, Lieberman P. Value of a second dose of epinephrine during anaphylaxis: a patient/caregiver survey. J Allergy Clin Immunol Pract. 2018;6(5):1559-67. https://doi.org/10.1016/j.jaip.2018.01.019