Vol. 24 - Num. 96
Original Papers
Urinary tract infections: etiology and antimicrobial susceptibilities
Francisco Miguel Escandell Ricoa, Lucía Pérez Fernándezb
aProfesor. Departamento de Enfermería. Universidad de Alicante. Alicante. España.
bCoordinadora de Enfermería. CS de Almoradí. Almoradí. Alicante. España.
Correspondence: FM Escandell . E-mail: francisco.escandell@ua.es
Reference of this article: Escandell Rico FM, Pérez Fernández L. Urinary tract infections: etiology and antimicrobial susceptibilities . Rev Pediatr Aten Primaria. 2022;24:e355-e362.
Published in Internet: 16-12-2022 - Visits: 37644
Abstract
Introduction: urinary tract infection (UTI) is one of the most prevalent diseases in clinical practice. In order to improve empirical treatment, the etiology of pediatric urinary tract infections and the antibiotic sensitivity profile of the responsible microorganisms have been studied.
Material and methods: cross-sectional, descriptive and retrospective study (2020-2021) in which isolated microorganisms with significant counts in urine samples from patients with UTI were included. The global etiology and according to age and sex were analyzed. Only one urine sample per patient and UTI episode was considered.
Results: Escherichia coli was the most isolated microorganism both in the population as a whole (62%) and in each of the groups analyzed according to age and sex. It was isolated in women significantly higher than in men (χ², p=0.043). Their sensitivity was: 94% fosfomycin and 86% amoxicillin-clavulanic acid.
Conclusions: Escherichia coli continues to be the most frequently isolated microorganism in UTI. Therefore, for low UTIs, amoxicillin-clavulanic acid and nitrofurantoin could be suitable options. Our environment should recommend fosfomycin, since it shows several advantages for its use, such as once-daily dosing, low side effects, adequate clinical and microbiological results, and little effect on the intestinal microbiota.
Keywords
● Escherichia coli ● Fosfomycin ● Microbial sensitivity tests ● Urinary tract infectionINTRODUCTION
Urinary tract infections (UTI) are among the most frequent bacterial infections in paediatrics, as 8-10% of girls and 2-3% of boys will have a symptomatic UTI by age 7 years.1 The overall prevalence of UTI in children under 2 years is approximately 5%.2-4 The frequency of recurrence before age 1 year is nearly 75% in boys, and after the first year of life, 40% of girls and 30% of boys have recurrent UTIs.5
In most cases, the causative agent is one of the bacteria that commonly colonise the perineal region in girls and the subpreputial space in boys. In children aged less than 2 years with UTI, fever is indicative of involvement of the renal parenchyma, so the risk of impaired renal function is greater in this age group.6,7
The most frequent causative agent of UTI is Escherichia coli, responsible for more than 75% of total cases and nearly 90% of uncomplicated cases. Some reviews show that the proportion of cases has decreased to 54-67% as the incidence of other causative microorganisms, such as Klebsiella, Proteus, Enterobacter, Citrobacter or Pseudomonas, has increased, especially in patients previously exposed to antibiotherapy or with genitourinary anomalies.8
Timely diagnosis and appropriate treatment of a UTI can prevent short-term complications, such as severe pyelonephritis or urosepsis, which develop in up to 30% of neonates and 20% of infants aged less than 3 months.9 In infants and young children, the signs and symptoms of UTI are nonspecific. Fever without source is usually the sole manifestation at onset of renal infection, which poses a challenge to early diagnosis and may prompt unnecessary treatment in children who are not at risk.10,11
The aim of our study was to describe the aetiology of UTIs in paediatrics and the antimicrobial susceptibility profile of the causative bacteria in Department of Health 21, which corresponds to a rural area in the southern Mediterranean coast of Spain.
MATERIAL AND METHODS
Study design and setting
We conducted a retrospective cross-sectional and descriptive study from January 1, 2020 to December 31, 2021 in which we included all microorganisms isolated with a significant colony count from the urine of patients with UTI. The analysis included only one urine sample per patient and UTI episode. Department of Health 21 offers paediatric care to 27 000 inhabitants. We analysed the causative agents of UTI overall, by age group (< 3 months, 3-6 months, 6-12 months, 12-18 months and 18-24 months) and by sex.
Protocol
Cultures were done by seeding urine in chromogenic medium. Microorganisms were identified based on the macroscopic appearance of the colonies in chromogenic medium and mass spectrometry. Antimicrobial susceptibility was assessed with the Kirby-Bauer diffusion method in Mueller-Hinton agar. The inclusion of antibiotics, phenotypic tests for detection of mechanisms of resistance and the interpretation of susceptibility test results adhered to the recommendations of the Clinical and Laboratory Standards Institute.12
Data were retrieved from the electronic health records system (Abucasis, Orion clinic, Gestlab) and collected in an electronic form that only the principal investigator could access, and all personal data were omitted. At all times, participants were identified through codes assigned specifically for the study, all of which started with “ITUP” followed by 3 digits (ITUP 001, ITUP 002…).
Statistical analysis
We conducted a descriptive analysis of all the variables, calculating absolute frequencies and percentages. We used the chi-square test for categorical variables. The software applications used in the study were Excel for the database and the SPSS statistical package (version 25.0; IBM Corp., Armonk, NY), and results were considered significant if p <0,05.
Ethical considerations
We sought the approval to perform the study of the Ethics and Research Committee of Department of Health 21 (file no. PI-2022-015).
The study adhered to the ethical principles for medical research in humans established in the Declaration of Helsinki at the 18th World Health Assembly of June 1964 as the guide to participation in the study. We also safeguarded confidentiality and the use of patient data adhered to Organic Law 3/2018, of December 5, on the protection of personal data and guarantee of digital rights. The study was exempt from informed consent from patients, as it was a retrospective epidemiological study that used secondary sources of information. The data were protected from unwarranted access by individuals not involved in the research project and therefore were treated as strictly confidential. The confidentiality of patients was safeguarded throughout the data processing and analysis.
RESULTS
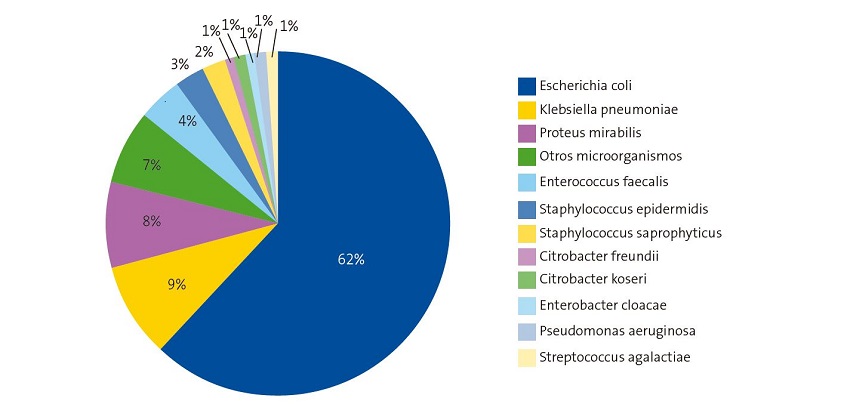
In the period under study, 388 uropathogens were isolated. Of this total, 275 (71%) were isolated in girls. Figure 1 shows the aetiology of UTIs in the study sample.
Table 1 presents the frequency of isolation of the most common microorganisms by age and sex. The frequency of UTI was higher in girls and decreased with age.
| Table 1. Urinary tract infections: aetiology and antimicrobial susceptibility. Distribution of uropathogens by patient sex and age (study period: 2020-2021) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boys | |||||||||||||||
| Microorganism |
<3 months n = 51 |
Microorganism |
3-6 months n = 20 |
Microorganism |
6-12 months n = 20 |
Microorganism |
12-18 months n = 15 |
Microorganism |
18-24 months n = 7 |
||||||
| n | % | n | % | n | % | n | % | n | % | ||||||
| Escherichia coli | 17 | 33.3 | Escherichia coli | 8 | 40 | Escherichia coli | 13 | 65 | Escherichia coli | 7 | 46.6 | Escherichia coli | 3 | 43 | |
| Proteus mirabilis | 12 | 23.5 | Klebsiella pneumoniae | 3 | 15 | Klebsiella pneumoniae | 2 | 10 | Proteus mirabilis | 3 | 20 | Proteus mirabilis | 2 | 28.5 | |
| Klebsiella pneumoniae | 8 | 15.6 | Klebsiella oxytoca | 2 | 10 | Proteus mirabilis | 1 | 5 | Enterococcus faecalis | 2 | 13.3 | Pseudomonas aeruginosa | 1 | 14.3 | |
| Enterococcus faecalis | 4 | 7.8 | Enterococcus faecalis | 1 | 5 | Enterococcus faecalis | 1 | 5 | Morganella morganii | 1 | 6.6 | Klebsiella pneumoniae | 1 | 14.3 | |
| Pseudomonas aeruginosa | 2 | 4 | Pseudomonas aeruginosa | 1 | 5 | Klebsiella oxytoca | 1 | 5 | |||||||
| Klebsiella oxytoca | 1 | 2 | Proteus mirabilis | 1 | 5 | Enterobacter cloacae | 1 | 5 | |||||||
| Enterobacter cloacae | 1 | 2 | Enterobacter cloacae | 1 | 5 | ||||||||||
| Citrobacter Koseri | 1 | 2 | Citrobacter Koseri | 1 | 5 | ||||||||||
| Staphylococcus saprophyticus | 1 | 2 | |||||||||||||
| Other microorganism* | 4 | 7.8 | Other microorganism* | 2 | 10 | Other microorganism* | 1 | 5 | Other microorganism* | 2 | 13.3 | ||||
| Girls | |||||||||||||||
| Microorganism |
<3 months n = 196 |
Microorganism |
3-6 months n = 30 |
Microorganism |
6-12 months n = 15 |
Microorganism |
12-18 months n = 16 |
Microorganism |
18-24 months n = 18 |
||||||
| n | % | n | % | n | % | n | % | n | % | ||||||
| Escherichia coli | 147 | 75 | Escherichia coli | 18 | 60 | Escherichia coli | 9 | 60 | Escherichia coli | 12 | 75 | Escherichia coli | 10 | 55.5 | |
| Klebsiella pneumoniae | 13 | 6.6 | Klebsiella pneumoniae | 3 | 10 | Klebsiella pneumoniae | 1 | 6.6 | Proteus mirabilis | 2 | 12.5 | Proteus mirabilis | 6 | 33.3 | |
| Klebsiella oxytoca | 13 | 6.6 | Enterococcus faecalis | 3 | 10 | Citrobacter Koseri | 1 | 6.6 | Klebsiella pneumoniae | 1 | 6.2 | Klebsiella pneumoniae | 2 | 11.1 | |
| Enterococcus faecalis | 7 | 3.4 | Pseudomonas aeruginosa | 1 | 3.3 | Enterococcus faecalis | 1 | 6.6 | Morganella morganii | 1 | 6.2 | ||||
| Staphylococcus saprophyticus | 5 | 2.5 | Proteus mirabilis | 1 | 3.3 | ||||||||||
| Proteus mirabilis | 4 | 2 | Staphylococcus saprophyticus | 1 | 3.3 | ||||||||||
| Morganella morganii | 3 | 1.4 | |||||||||||||
| Enterobacter cloacae | 2 | 1 | |||||||||||||
| Pseudomonas aeruginosa | 1 | 0.5 | |||||||||||||
| Streptococcus agalactiae | 1 | 0.5 | |||||||||||||
| Other microorganism * | 1 | 0.5 | Other microorganism * | 3 | 10 | Other microorganism * | 3 | 20 | |||||||

E. coli (62%) was the most frequently isolated microorganism in the total sample, in both sexes and in each age group under consideration (Figure 1 and Table 1). We found significant differences in the frequency of isolation based on age and sex, ranging from 75% (girls aged less than 3 months) and 33.3% (boys aged less than 3 months). Comparing the frequency of E. coli isolation based on sex and age, we found that the percentage of isolation was significantly higher in girls compared to boys (χ2, p = 0.043) and that it decreased with age. Klebsiella pneumoniae and Proteus mirabilis were next in frequency.
In the group aged 0 to 6 months, Enterococcus faecalis and Pseudomonas aeruginosa were isolated more frequently compared to other age groups (Table 1).
Of the total isolates, 1.5% corresponded to Staphylococcus saprophyticus, with a higher proportion in girls (5.8%). Morganella morganii and Citrobacter koseri were isolated less frequently in both sexes and all age groups.
As regards the antimicrobial susceptibility of the most frequently isolated uropathogens, we found a broad range in susceptibility in E. coli, from 86% of isolates susceptible to amoxicillin-clavulanic acid to 94% susceptible to fosfomycin.
Table 2 shows the overall susceptibility rate for each antibiotic in 2021, calculated based on the frequency of isolation of each microorganism, patient age and sex. We found a higher rate of susceptibility in isolates from girls compared to boys. When it came to the susceptibility of antibiotics chiefly used to treat UTIs, more than 95% of isolates in girls were susceptible to fosfomycin compared to 92% in boys, while more than 89% of isolates in girls and more than 86% in boys were susceptible to nitrofurantoin.
| Table 2. Urinary tract infections: aetiology and antimicrobial susceptibility. Antimicrobial susceptibility (%) calculated based on the frequency of isolates by sex and age group (year 2021) | |||||
|---|---|---|---|---|---|
| Boys | |||||
| Antibiotic agent |
<3 months n = 51 |
3-6 months n = 20 |
6-12 months n = 20 |
12-18 months n = 15 |
18-24 months n = 7 |
| Amoxicillin-clavulanic acid | 82.3 | 75 | 95 | 80 | 71.4 |
| Cefuroxime | 76.4 | 70 | 90 | 73.3 | 71.4 |
| Cefixime | 59 | 60 | 85 | 40 | 57.1 |
| Norfloxacin | 51 | 35 | 45 | 40 | 57.1 |
| Ciprofloxacin | 43 | 35 | 45 | 40 | 51.4 |
| Fosfomycin | 94.1 | 85 | 100 | 100 | 85.7 |
| Nitrofurantoin | 88.2 | 80 | 100 | 100 | 71.4 |
| Girls | |||||
| Antibiotic agent |
<3 months n = 196 |
3-6 months n = 30 |
6-12 months n = 15 |
12-18 months n = 16 |
18-24 months n = 18 |
| Amoxicillin-clavulanic acid | 90.8 | 83.3 | 93.3 | 100 | 88.8 |
| Cefuroxime | 88.2 | 76.6 | 66.6 | 100 | 77.7 |
| Cefixime | 59.6 | 66.6 | 60 | 62.5 | 66.6 |
| Norfloxacin | 60.2 | 56.6 | 53.3 | 56.2 | 66.6 |
| Ciprofloxacin | 51 | 70 | 73.3 | 25 | 61.1 |
| Fosfomycin | 94.3 | 96.6 | 100 | 100 | 88.8 |
| Nitrofurantoin | 88.2 | 96.6 | 86.6 | 100 | 83.3 |

DISCUSSION
Our study contributes information on the aetiology of paediatric urinary tract infections and the antimicrobial susceptibility profile of the causative pathogens identified in Department of Health 21, which covers a rural area in the southern Mediterranean.
In our area, in agreement with studies conducted outside Spain, 13 we found that the most frequent causative agent was E. coli (62%), with a higher prevalence in female patients, as reported by other authors.14 Following E. coli, the second leading group of UTI causative bacteria in terms of frequency of isolation were other Enterobacteriaceae (38%). Other studies have found that the frequency of isolation of each species of Enterobacteriaceae depends on the study period, population, geographic location, patient age, recurrence of infection, presence of urinary system pathology and/or previous antibiotherapy.12,15
In agreement with previous studies,16 Proteus mirabilis was more common in boys than girls. The second most frequently isolated pathogen was Klebsiella pneumoniae (9%), with a relatively uniform distribution both by sex and age group. Enterococcus faecalis was also isolated frequently (4%), as reported in the previous literature,17 which mentions risk factors associated with the host and the use of antibiotherapy.18
Isolation of Staphylococcus saprophyticus (2%) is more frequent in girls and varies between series, depending on the population under study. Our findings were consistent with previous studies in that Staphylococcus saprophyticus was a more frequent uropathogen in girls.19,20 Therefore, it is essential to be aware of Staphylococcus saprophyticus as a possible aetiological agent in girls to guide the appropriate selection of empiric antibiotherapy, as resistance patterns may be different in this bacterium.21
The results of our study show that, overall, the proportion of pathogens causing UTI susceptible to amoxicillin-clavulanic acid was 86%, a percentage that allows its use for empiric treatment. Quinolones are widely used in patients with acute infections, complicated or uncomplicated. Despite that there are studies confirming that Spain is among the European countries with the highest percentages of quinolone-resistant E. coli,22 quinolones appear to be at least as effective as cotrimoxazole and more effective that some beta-lactam antibiotics.23 In our study, the overall percentage of isolates of UTI-causing microorganisms susceptible to quinolones was 52%, with evidence of a higher activity of second-generation quinolones (norfloxacin) against gram-negative bacteria, as observed in previous studies.24
The Infectious Diseases Society of America (IDSA) guidelines published in 2022 state that empiric treatment decisions should be guided by local data for the most probable pathogens, the severity of illness, the likely source of infection and other specific individual factors. It is also important to differentiate between bacterial colonization and infection, as unnecessary antibiotherapy will only promote the development of drug resistance and may also cause avoidable harm to the patient associated with its use.25
When it comes to antibiotic prophylaxis, local antimicrobial resistance patterns should be taken into account, and the selected agent should be the one with the narrowest possible spectrum to prevent the development of drug resistance.26 Our findings, as do those of other studies, support the recommendation of nitrofurantoin as an effective treatment for uncomplicated UTIs.27
In contrast with the previously discussed antibiotic agents, more than 90% of E. coli isolates continued to be susceptible to fosfomycin and nitrofurantoin. In agreement with previous studies, we consider that these drugs could be very useful for empiric treatment of uncomplicated UTI.28 Another multicentre study conducted in Spain found that the percentage of E. coli isolates susceptible to fosfomycin varied from 95.6 to 99.4% in the different autonomous communities of Spain included in the study.29
One possible limitation of the study is its design and the recruitment of the sample from a single Department of Health. Performance of prospective studies would be useful. One of the strengths of the study is that it includes the annual data on community-acquired UTI in a whole Department of Health, which are indispensable for delivery of up-to-date empiric antibiotherapy, reviewing and updating clinical practice guidelines for UTI and as the foundation of any antimicrobial stewardship programme (ASP). This information is essential for adequate treatment of infections and also to prevent treatment failure and the development of antimicrobial resistance. Local epidemiological studies must be carried out at regular intervals to update these data and analyse temporal trends in the development of antimicrobial resistance.
CONCLUSION
Antimicrobial susceptibility profiles have been changing over time, between health care areas and even between community settings, so treatment recommendations cannot be universal, but must be based on local susceptibility data for the most common pathogens. Additional aspects should also be considered, such as the efficacy and safety of the drug, the cost and duration of treatment, the ease of administration and its potential for selection of resistant microorganisms.
In conclusion, E. coli continues to be the organism isolated most frequently in UTIs, with 90% of isolates found susceptible to fosfomycin and 86% susceptible to amoxicillin-clavulanic acid. Therefore, in amoxicillin-clavulanic acid and nitrofurantoin could be suitable options for treatment of lower tract UTIs. In our region, fosfomycin should be recommended, as it offers certain advantages, such as a single dose a day, few side effects, adequate clinical and microbiological outcomes and a limited impact on the intestinal microbiota.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article.
ABBREVIATIONS
ASP: antimicrobial stewardship programme · UTI: urinary tract infection.
ETHICAL CONSIDERATIONS
The study was approved by the Research Ethics Committee of Department of Health 21 (file no. PI-2022-015).
REFERENCES
- Hellström A, Hanson E, Hansson S, Hjälmas K, Jodal U. Association between urinary symptoms at 7 years old and previous urinary tract infection. Arch Dis Child. 1991;66:232- 4.
- Baraff LJ. Management of fever without source in infants and children. Ann Emerg Med. 2000;36:602-14.
- Mintegi S, González M, Pérez A, Pijoán JI, Capapé S, Benito J. Infants aged 3-24 months with fever without source in the emergency room: characteristics, management and outcome. An Pediatr (Barc). 2005;62:522-8.
- Lee GM, Fleisher GR, Harper MB. Management of febrile children in the age of the conjugate pneumococcal vaccine: a cost-effectiveness analysis. Pediatrics. 2001;108:835-44.
- Mattoo TK. Are prophylactic antibiotics indicated after a urinary tract infection? Curr Opin Pediatr. 2009;21:203-6.
- Coulthard MG, Lambert HJ, Vernon SJ, Hunter EW, Keir MJ, Matthews JN. Does prompt treatment of urinary tract infection in preschool children prevent renal scarring: mixed retrospective and prospective audits. Arch Dis Child. 2014;99:342-7.
- Shaikh N, Ewing AL, Bhatnagar S, Hoberman A. Risk of renal scarring in children with a first urinary tract infection: A systematic review. Pediatrics. 2010;126:1084-91.
- Alberici I, Bayazit AK, Drozdz D, Emre S, Fischbach M, Harambat J, et al. Pathogens causing urinary tract infections in infants: a European overview by the ESCAPE study group. Eur J Pediatr. 2014;174:783-90.
- Bhat RG, Katy TA, Place FC. Pediatric urinary tract infections. Emerg Med Clin North Am. 2011;29:637-53.
- Pinzón Fernández MV, Zúñiga Cerón LF, Saavedra Torres JS. Infección del tracto urinario en niños, una de las enfermedades infecciosas más prevalentes. Rev Fac Med. 2018;66:393-8.
- Kaufman J, Temple-Smith M, Sanci l. Urinary tract infections in children: an overview of diagnosis and management. BMJ Paediatr Open. 2019;3:e000487.
- Anesi JA, Lautenbach E, Nachamkin I, Garrigan C, Bilker WB, Omorogbe J, et al. The role of extended-spectrum cephalosporin-resistance in recurrent community-onset Enterobacteriaceae urinary tract infections: a retrospective cohort study. BMC Infect Dis. 2019;19:163.
- Leung AKC, Wong AHC, Leung AAM, Hon KL. Urinary tract infection in children. Recent Pat Inflamm Allergy Drug Discov. 2019;13:2-18.
- Samancı S, Çelik M, Köşker M. Antibiotic resistance in childhood urinary tract infections: A single-center experience. Turk Pediatri Ars. 2020;55:386-92.
- Sorlozano A, Jiménez Pacheco A, De Dios Luna del Castillo J, Sampedro A, Martínez-Brocal A, Miranda Casas C, et al. Evolution of the resistance to antibiotics of bacteria involved in urinary tract infections: a 7-year surveillance study. Am J Infect Control. 2014;42:1033-8.
- García Vera C. Infecciones urinarias. Rev Pediatr Aten Primaria. Supl. 2013;(22):71-80.
- Tripathi A, Shukla S, Singh A, Prasad K. Prevalencia, resultado y factor de riesgo asociado con Enterococcus faecalis y Enterococcus faecium resistentes a la vancomicina en un hospital de atención terciaria en el norte de la India. Indian J Med Microbiol. 2016;34:38-45.
- Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369:1883-91.
- Lo DS, Shieh HH, Barreira ER, Ragazzi SL, Gilio AE. High frequency of Staphylococcus saprophyticus urinary tract infections among female adolescents. Pediatr Infect Dis J. 2015;34:1023-5.
- Palou J, Pigrau C, Molina I, Ledesma JM, Angulo J; Grupo Colaborador Español del Estudio ARESC. Etiología y sensibilidad de los uropatógenos identificados en infecciones urinarias bajas no complicadas de la mujer (Estudio ARESC): implicaciones en la terapia empírica. Med Clin (Barc). 2011;136:1-7.
- Eriksson A, Giske CG, Ternhag A. La importancia relativa de Staphylococcus saprophyticus como patógeno del tracto urinario: distribución de bacterias entre las muestras de orina analizadas durante 1 año en un importante laboratorio sueco. APMIS. 2013;121:72-8.
- Schito GC, Naber KG, Botto H, Palou J, Mazzei T, Gualco l, et al. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Agents. 2009;34:407-13.
- Hooton TM, Scholes D, Gupta K, Stapleton AE, Roberts PL, Stamm WE. Amoxicillin-clavulanate vs ciprofloxacin for the treatment of uncomplicated cystitis in women: a randomized trial. JAMA. 2005;293:949-55.
- Alós JI. Quinolonas. Enferm Infecc Microbiol Clin. 2009;27:290-7.
- Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin Infect Dis. 2021;72:e169-e183.
- Hawkey PM, Jones AM. The changing epidemiology of resistance. J Antimicrob Chemother. 2009;64:3-10.
- Zhanel GG, Hisanaga TL, Laing NM, DeCorby MR, Nichol KA, Palatnik LP, et al. Antibiotic resistance in outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int J Antimicrob Agents. 2005;26:380-8.
- Fasugba O, Mitchell BG, Mnatzaganian G, Das A, Collignon P, Gardner A. Five-year antimicrobial resistance patterns of urinary Escherichia coli at an Australian tertiary hospital: time series analyses of prevalence data. PLoS One. 2016;11:e0164306.
- Andreu A, Planells I; Grupo Cooperativo Español para el Estudio de la Sensibilidad Antimicrobiana de los Patógenos Urinarios. Etiología de la infección urinaria baja adquirida en la comunidad y resistencia de Escherichia coli a los antimicrobianos de primera línea. Estudio nacional multicéntrico. Med Clin (Barc). 2008;130:481-6.