Vol. 24 - Num. 96
Original Papers
Ondansetron for the control of vomiting associated with acute gastritis/gastroenteritis in Pediatric Emergencies: use, abuse and appropriate use
Alicia García Marína, Germán Lloret Ferrándizb, Javier González de Diosc
aFacultad de Medicina. Universidad Miguel Hernández. Alicante. España.
bServicio de Pediatría. Hospital General Universitario de Alicante. Alicante. ISABIAL-Instituto de Investigación Sanitaria y Biomédica de Alicante. España.
cServicio de Pediatría. Hospital General Universitario de Alicante. Departamento de Pediatría. Universidad Miguel Hernández. ISABIAL-Instituto de Investigación Sanitaria y Biomédica. Alicante. España.
Correspondence: J González. E-mail: javier.gonzalezdedios@gmail.com
Reference of this article: García Marín A, Lloret Ferrándiz G, González de Dios J. Ondansetron for the control of vomiting associated with acute gastritis/gastroenteritis in Pediatric Emergencies: use, abuse and appropriate use . Rev Pediatr Aten Primaria. 2022;24:351-61.
Published in Internet: 22-11-2022 - Visits: 34112
Abstract
Background: ondansetron is an antiemetic widely used in clinical practice for the control of vomiting associated with gastritis and/or acute gastroenteritis in children. However, the available evidence about its use is controversial, its directions for use are not clearly defined and there is no unanimity on its use in clinical practice guidelines.
Methodology: we performed a retrospective cohort study which included a total of 825 children between 0 and 14 years, who presented symptoms of vomiting associated with gastritis and/or acute gastroenteritis and attended the Pediatric Emergency Department of a tertiary hospital in 2019. The association between the use of ondansetron and the need for intravenous rehydration, hospitalisation, length of stay in the Pediatric Emergency Department and return visits to the emergency department within 72 hours was analysed.
Results: of the sample studied, 38.7% of the patients received ondansetron. The administration of ondansetron reduced the risk of hospital admission (OR 0.19; 95% CI 0.04 to 0.84) and decreased the length of stay in the emergency department (p = 0.000). No significant differences were found in reducing the need for intravenous rehydration (OR 0.65; 95% CI 0.40 to 1.05) or in return visits to emergency department within 3 days (OR 1.38; 95% CI 0.82-2.31).
Conclusions: our results suggest that the use of ondansetron could be beneficial in children older than 6 months with vomiting associated with gastritis and/or acute gastroenteritis and with mild-to-moderate dehydration.
Keywords
● Acute gastroenteritis ● Children ● Ondansetron ● VomitingINTRODUCTION
Acute gastroenteritis (AGE) is the second most frequent reason for visits to the paediatric emergency department (PED) in developed countries.1-3 Vomiting is also a common presenting complaint in the PED4, and may manifest in isolation (gastritis) or associated with AGE, constituting an important component of the latter.3,5-7 The problem with vomiting is that it makes oral rehydration difficult. This is where antiemetic drugs come into play, facilitating oral rehydration, which is less invasive than intravenous rehydration.3,6
Ondansetron is the only antiemetic drug that has proven efficacious in reducing vomiting associated with gastritis/AGE in children,2 and there is no scientific evidence of sufficient quality to support the use of other antiemetic drugs in these cases.5,8,9 Currently, ondansetron is indicated for treatment of vomiting associated with chemotherapy, radiation therapy or after surgery in children aged more than 6 months.10 However, in clinical practice it is used for treatment of vomiting secondary to gastritis/AGE, although its routine use is not recommended.11
At the same time, several studies suggest that children with vomiting associated with gastritis/AGE and with mild to moderate dehydration may benefit from ondansetron, as it reduces the frequency of vomiting and facilitates oral rehydration.12-14 However, several studies have found an increase in the episodes of diarrhoea observed in patients treated with ondansetron,13-15 which could contribute to an increase in subsequent visits to the PED and to dehydration. Another adverse effect associated with its use is a prolonged QT interval.2,10,16
In addition, some studies have concluded that ondansetron decreases the frequency of vomiting, reduces the need of intravenous rehydration and decreases the length of stay in the PED, but does not change the admission rate or the frequency of return PED visits.6,17 However, the published data are contradictory, as there are also studies that suggest that its use decreases the frequency of return visits to the PED and the rate of admission, and even that it does not decrease the need of intravenous fluid rehydration.18-20
Some guidelines recommend the use of ondansetron to manage vomiting in children with gastritis/AGE, such as those of the Canadian Pediatric Society, although not routinely if diarrhoea is the main symptom, as diarrhoea is the most frequent adverse event.21 Other guidelines, such as the one by the National Institute for Health and Care Excellence (NICE), do not recommend the use of antiemetic drugs to manage vomiting associated with AGE.22 On the other hand, the guideline of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) does also not recommend the routine use of ondansetron, but notes that it could be effective and that further research on the subject is required.2 Notwithstanding, ondansetron is widely used in clinical practice.23
Thus, given the current debate regarding the use of ondansetron, there is no consensus among the different clinical practice guidelines. The indications of ondansetron are clearly established for vomiting associated with chemotherapy or radiotherapy or after surgery, but not in the case of gastritis/AGE, in which its use is left to the judgment of the physician. The objectives of our study were the following: our primary objectives were to assess the patterns of use of ondansetron in the PED of a tertiary care hospital in children aged 0 to 14 years (both included) with vomiting associated with gastritis/AGE and to propose indications based on the best available evidence after reviewing the scientific literature; our secondary objectives were to analyse the need of intravenous rehydration, hospital admission, length of stay in the PED and return visits within 72 horas in patients who received ondansetron compared to patients who received other treatments.
MATERIAL AND METHODS
Study design and sample selection
We conducted a retrospective observational cohort study through the review of the electronic health records of a sample of patients aged 0 to 14 years (both included) with vomiting associated with gastritis/AGE who visited the PED of the tertiary care hospital in 2019.
After analysing the data, we found that in 2019 a total of 37 617 children were managed in the PED, of who 3645 (9.68%) had a diagnosis of vomiting and/or AGE; the distribution by month of these visits is shown in Table 1. To limit the sample size, we collected data for the patients who visited the PED on the Tuesdays and Saturdays of the odd weeks and the Thursdays and Sundays of the even weeks of 2019.
| Table 1. Monthly distribution of cases of vomiting and/or acute gastroenteritis in 2019 in the paediatric emergency department (PED) of the Hospital Universitario de Alicante | ||||
|---|---|---|---|---|
| Cases | Cases/day | PED visits | % PED visits | |
| January | 266 | 8.58 | 3513 | 7.57% |
| February | 295 | 10.53 | 3329 | 8.86% |
| March | 423 | 13.64 | 3536 | 11.96% |
| April | 298 | 9.93 | 2982 | 9.99% |
| May | 210 | 6.77 | 2825 | 7.43% |
| June | 254 | 8.46 | 2691 | 9.43% |
| July | 309 | 9.96 | 2677 | 11.54% |
| August | 243 | 7.83 | 2681 | 9.06% |
| September | 275 | 9.16 | 2772 | 9.92% |
| October | 371 | 11.96 | 3576 | 10.37% |
| November | 384 | 12.8 | 3228 | 11.89% |
| December | 317 | 10.22 | 3807 | 8.32% |
| TOTAL | 3645 | 9.98 | 37 617 | 9.68% |

We excluded any patient with vomiting for a cause other than gastritis/AGE, and patients with gastritis/AGE without associated vomiting, which yielded a final sample of 825 patients. Information on the patients excluded from the study can be found in Table 2.
| Figure 1. Flow chart of patient selection |
|---|
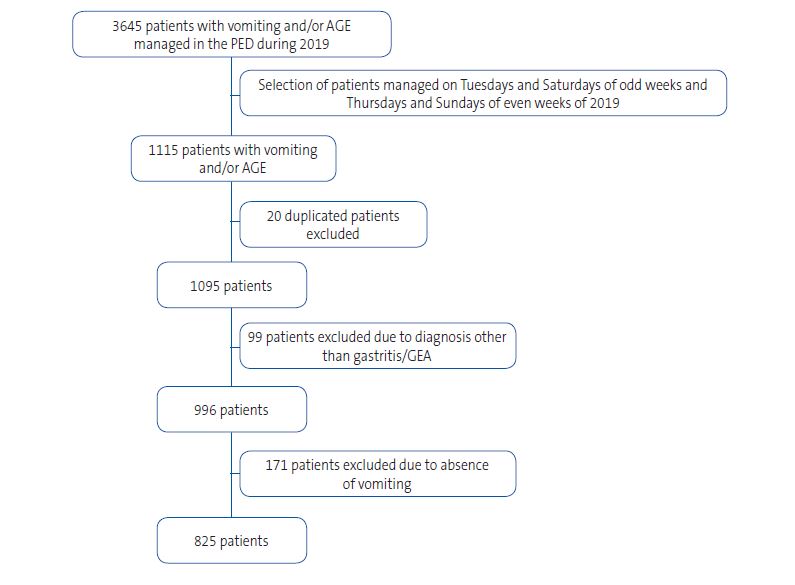 |

| Table 2. Diagnoses of patients excluded from the study | |
|---|---|
| Diagnosis | n |
| Upper respiratory tract infection | 44 |
| Cutaneous complaints | 9 |
| Trauma | 6 |
| Ingestion of objects or products | 5 |
| Herpangina-oral aphthae | 3 |
| Headache | 2 |
| Recent surgery | 2 |
| Abdominal pain | 2 |
| Lymphoblastic leukaemia | 2 |
| Presyncope | 2 |
| Haematemesis | 2 |
| Anal fissure | 2 |
| Mesenteric lymphadenitis | 2 |
| Ophthalmological complaints | 2 |
| Febrile syndrome | 1 |
| Acute rhinitis | 1 |
| Bronchiolitis | 1 |
| Febrile seizure | 1 |
| Benzodiazepine poisoning | 1 |
| Inguinal hernia | 1 |
| Absence seizure | 1 |
| Acute choking | 1 |
| Ventriculoperitoneal shunt carrier | 1 |
| Gastro-oesophageal reflux | 1 |
| Mallory-Weiss syndrome | 1 |
| Evaluation of hepatomegaly | 1 |
| Resolved vomiting | 1 |
| Suspected diarrhoea caused by amoxicillin-clavulanic acid | 1 |

In the PED, ondansetron was always administered via the sublingual route at a dose standardised by weight interval: 2 mg in patients weighing 15 kg, 4 mg for those weighing 15 to 30 kg, and 8 mg for those weighing more than 30 kg.24
Study variables
Based on the list of patients provided by the Department of Admissions and Records of the hospital, we collected data by reviewing the electronic health records, using the software Orion Clinic® version 11.0 (Agencia Valenciana de Salud, Spain). We entered the data in a spreadsheet (Microsoft Excel, version 16.0; Microsoft®, USA) for subsequent analysis. All the data were anonymised.
We collected clinical and epidemiological data on the patients, including age, sex, body temperature and blood glucose and ketone levels. The variables related to clinical manifestations were abdominal pain, presence or absence of dehydration, number of vomiting episodes and liquid stools and the time from onset. To determine the level of dehydration based on the information available in the health records, we used the Gorelick scale.25 The variables related to the treatment given to the patient were the sublingual administration of ondansetron sublingual, oral rehydration and/or intravenous rehydration.
We also collected data on the length of stay in the PED (time elapsed from arrival to PED to discharge or hospital admission), return visits within 72 hours (excluding any visits to the primary care centre or paediatrician of the catchment area) and hospital admissions.
Statistical analysis
The statistical analysis was conducted with the software IBM SPSS® Statistics for Windows, version 25.0. To identify significant differences between the groups under study, we used the Pearson χ2 test for qualitative variables and the Student t test or Mann-Whitney U test for quantitative variables, depending on whether or not the data followed a normal distribution. The test used to assess for normality was the Shapiro-Wilks test. For categorical variables, we assessed association by means of odds ratios (ORs) with their 95% confidence intervals (CIs). In every test, a p-value of less than 0.05 was considered statistically significant.
Ethical considerations
The study was conducted in adherence with the requirements of the Ethics Committee of the hospital. The committee approved the exemption from informed consent, and the data were anonymised and protected at all times.
RESULTS
Of the total of patients included in the study (n = 825), 320 (38.79%) received ondansetron sublingually. Tables 3 and 4 summarise the characteristics of the study sample.
| Table 3. Characteristics of the sample (quantitative variables) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ondansetron(n = 320) | No ondansetron (n = 505) | ||||||||
| Variables | N valid (%) | Median | Mean (SD) | (min; max) | N valid (%) | Median | Mean (SD) | (min; max) | p value |
| Age (years) | 320 | 4.73 | 5.73 (3.75) | (0.3; 14.9) | 505 | 2.81 | 4.19 (3.61) | (0.02; 14.9) | 0.000 |
| <1 year | 7 (2.2) | 95 (18.8) | |||||||
| 1.1-5 years | 160 (50.0) | 256 (50.7) | |||||||
| 5.1-10 years | 100 (31.3) | 107 (21.2) | |||||||
| 10.1-15 years | 53 (16.6) | 47 (9.3) | |||||||
| Weight (kg) | 319 | 18.3 | 22.97 (13.7) | (7.15;92) | 500 | 14.7 | 19.05 (13.63) | (3.03; 84.5) | 0.000 |
| Temperature (°C) | 313 | 36.4 | 36.5 (0.9) | (34.5;40.2) | 502 | 36.5 | 36.63 (0.9) | (34.5; 39.7) | 0.023 |
| <37 °C | 241 (77.0) | 367 (73.1) | |||||||
| 37-38 °C | 49 (15.7) | 97 (19.3) | |||||||
| >38 °C | 23 (7.3) | 38 (7.6) | |||||||
| Blood glucose (mg/dL) | 173 | 101 | 101.44 (24.25) | (44;179) | 170 | 96 | 95.36 (26.21) | (31; 211) | 0.026 |
| <70 mg/dL | 19 (10.98) | 33 (19.41) | |||||||
| 70-160 mg/dL | 152 (87.86) | 135 (79.41) | |||||||
| >160 mg/dL | 2 (1.16) | 2 (1.18) | |||||||
| Blood ketones (mmol/L) | 100 | 1.4 | 2.0 (1.85) | (0.1;6.3) | 87 | 2.1 | 2.56 (2.08) | (0.1;6.9) | 0.057 |
| Number of vomiting episodes | 220 | 5 | 6.55 (4.55) | (1;35) | 347 | 3 | 4.012 (3.89) | (1;40) | 0.000 |
| Duration of vomiting (hours) | 311 | 8 | 15.77 (20.59) | (0;120) | 474 | 12 | 23.41 (25.4) | (0; 120) | 0.000 |
| Number of liquid stools | 274 | 0 | 1.64 (2.65) | (0;20) | 392 | 0 | 2.28 (4.3) | (0;32) | 0.000 |
| Duration of liquid stools (hours) | 306 | 0 | 9.4 (20.16) | (0;120) | 468 | 3.5 | 18.09 (27.92) | (0;120) | 0.000 |

| Table 4. Characteristics of the sample (qualitative variables) | |||
|---|---|---|---|
| Ondansetron (n = 320) (%) |
No ondansetron (n = 505) (%) |
||
| Variables | N (%) | N (%) | p value |
| Sex | 0.502 | ||
|
180 (56.3) | 272 (53.9) | |
| Abdominal pain | 131 (40.9) | 159 (31.5) | 0.006 |
| Dehydration | 19 (5.9) | 33 (6.5) | 0.730 |
|
19 (5.9) | 26 (5.1) | |
|
0 (0) | 6 (1.2) | |
|
0 (0) | 1 (0.2) | |
| Liquid stools | 126 (39.38) | 294 (58.22) | 0.000 |
| Oral rehydration attempted | 54 (16.87) | 266 (52.67) | 0.000 |

When we stratified patients by aged, we found that infants under 1 year clustered in the group that did not receive ondansetron (18.5% vs 2.2%). Only 52 patients (6.3%) had dehydration, and we did not find significant differences on this account between the two study groups (p = 0.730). In the group treated with ondansetron, only 19 patients (5.9%) had dehydration, which was mild in every case.
Eighty-nine patients (10.8%) received intravenous fluids for rehydration, and the administration of ondansetron was not significantly associated with a reduced need of intravenous fluids (OR 0.65; 95% CI: 0.40 to 1.05). However, a separate analysis of the small subset of patients with dehydration, revealed that intravenous rehydration was used less frequently in those who received ondansetron than in those who did not (OR 0.16; 95% CI: 0.04 to 0.57).
Only 2 patients (0.63%) required admission in the group treated with ondansetron and 16 (3.17%) in the group without ondansetron, showing a reduction in the risk of admission with the use of ondansetron (OR 0.19; 95% CI: 0.04 to 0.84).
Sixty-three patients (7.7%) returned to the PED within 72 hours. However, the administration of ondansetron was not significantly associated with return visits to the PED in 72 hours (OR 1.38; 95% CI: 0.82 to 2.31). The length of stay in the PED was significantly longer in the group that received ondansetron (p = 0.000) (Table 5).
| Table 5. Outcomes of interest | ||||||
|---|---|---|---|---|---|---|
| Primary and secondary outcomes | N (%) | Ondansetron (n = 320) (%) |
No ondansetron (n = 505) (%) |
p value | 95% CI | OR |
| IV rehydration | 89 (10.8) | 27 (8.44) | 62 (12.28) | 0.079 | 0.409-1.059 | 0.658 |
| IV rehydration in dehydrated patients (n = 52) | 35 (67.3) | (n = 19) (%) | (n = 33) (%) | 0.003 | 0.045-0.575 | 0.162 |
| 8 (42.11) | 27 (81.82) | |||||
| Hospital admission | 18 (2.18) | 2 (0.63) | 16 (3.17) | 0.008 | 0.044-0.842 | 0.192 |
| Return visit to PED (<72 hours) | 63 (7.7) | 29 (9.06) | 34 (6.73) | 0.224 | 0.824-2.314 | 1.381 |
| Length of PED stay (minutes) | 825 (100) | 320 (100) | 505 (100) | |||
|
116.5 | 56 | 0.000 | |||
|
162.46 (195.516) |
128.79 (188.926) |
||||
|
(14;1234) | (13;1185) | ||||

In a separate analysis removing infants aged less than 6 months (31 patients), we noticed that only 2 had received ondansetron, while the other 29 had not. Once these patients were removed, there still were no significant differences between groups in the use of intravenous rehydration (OR 0.68; 95% CI: 0.42 to 1.10) and the frequency of return visits to the PED within 72 hours (OR 1.29; 95% CI: 0.76 to 2.19). On the other hand, once these infants were removed from the sample, we only found 8 hospital admissions (1.68%) in the group that did not receive ondansetron, so that the difference was no longer statistically significant (p = 0.173).
When it came to the stay in the PED, there were still significant differences in the median length of stay between the two groups (p = 0.000) (Table 6).
| Table 6. Outcomes of interest after excluding infants aged less than 6 months from the analysis | ||||||
|---|---|---|---|---|---|---|
| Primary and secondary outcomes | N (%) | Ondansetron (n = 318) (%) |
No ondansetron (n = 476) (%) |
p value | 95% CI | OR |
| IV rehydration | 84 (10.58) | 27 (8.49) | 57 (11.97) | 0.113 | 0.421-1.104 | 0.680 |
| Hospital admission | 10 (1.26) | 2 (0.63) | 8 (1.68) | 0.173 | 0.07-1.775 | 0.370 |
| Return visit to PED (<72 hours) | 61 (7.68) | 28 (8.8) | 33 (6.93) | 0.335 | 0.767-2.191 | 1.296 |
| Length of PED stay (minutes) | 794 (100) | 318 (100) | 476 (100) | |||
|
116.5 | 54 | 0.000 | |||
|
162.29 | 123.53 | ||||
|
(14;1234) | (13;1185) | ||||

DISCUSSION
In this retrospective study, we found that a high proportion (38.7%) of patients with vomiting associated with gastritis/AGE received sublingual ondansetron. This was consistent with the findings of some previous studies, which evinced a sharp increase in the use of ondansetron for treatment of vomiting secondary to gastritis/AGE in recent years.26
To reduce the sample size and prevent biases in data collection, we randomly selected 2 days a week for each week of 2019 to ensure representation of every season, of weekdays and weekends, and every hour of the day. We did this to try to reduce the influence of various factors during specific times of the year, and to include a greater number of health care staff than we would have if we had randomly selected entire months.
Our findings suggest that ondansetron does not reduce the frequency of intravenous rehydration, which stood in contrast with the majority of studies in the previous literature.17,18,27 This could be due to differences between the populations under study, as most participants in our study (observational design, real-world clinical practice) did not have dehydration, whereas in other studies (experimental design) having dehydration of any degree was an inclusion criteria. Furthermore, in our study, a substantial number of patients without dehydration (301 patients) were treated with ondansetron. This could reflect an incorrect use of this drug, given that there has been no scientific evidence to date of any benefits of ondansetron in these patients,18,20 so that the inappropriate prescribing of ondansetron in our sample may have interfered with finding evidence of its potential benefit.
A randomised controlled trial conducted by Freedman et al. in 201920 in patients who were not dehydrated had similar findings in that ondansetron was not significantly associated with a reduction in the proportion of patients treated with intravenous rehydration. We think that this study may be similar to ours, as it only included patients without dehydration, given that an unexpected majority of patients in our study did not have dehydration (93.7%). In fact, analysing the subset of patients with dehydration in our study, we found that ondansetron was associated with a statistically significant reduction in the need of intravenous rehydration (OR 0.16; 95% CI: 0.04 a 0.57).
On the other hand, in a study conducted by Benary et al. published in 2020,19 more than half the patients that received intravenous fluids for rehydration (55.1%) had been treated previously with ondansetron. This contrasted with our findings, as most patients in our study managed with intravenous rehydration had not been treated with ondansetron (69.66%), which suggests either an excessive use of intravenous rehydration or an inappropriate use of ondansetron, as avoiding intravenous fluid therapy is supposedly one of the main objectives of using ondansetron.
The current evidence on the reduction in hospital admissions is contradictory. Some studies suggest that the use of ondansetron reduces the rate of hospitalization, which is consistent with the overall findings of our study.14,18 However, other studies did not find significant differences in hospitalization between patients treated and not treated with the drug,17,27 including ours, as once we excluded infants aged less than 6 months, we did not find differences between the two groups. This could be attributed to the sample selection, as most previous studies excluded infants under 6 months because ondansetron is not usually administered before that age. All of this suggests that any findings on the subject must be interpreted taking into account the population under study.
As regards return visits to the PED within 72 hours, we did not find significant differences. In our study, 7.6% of patients return to the PED, compared to approximately 4.7% in other studies.19 While this outcome could be important, as it could reflect treatment failure, we have to take into account the health literacy of the population under study and the accessibility of hospital emergency services in each country as possible factors contributing to this discrepancy.
Another study, conducted by Freedman et al.,17 found a 12% decrease in the length of stay in the PED in the group treated with ondansetron, with a mean duration of 106 minutes. Our findings differ, as the length of stay in minutes was longer in the group treated with ondansetron (162.46 vs. 128.79). This may be explained by different factors. The length of stay in the PED may be influenced by the time the patient was managed, as there is a tendency toward shorter stays in the PED when the patient visits the department at night. Furthermore, when ondansetron is administered, the patient is usually kept under observation for 30 minutes before resuming oral rehydration, which contributed to the length of stay in patients treated with the drug. In opposition, we suspect that in the group that was not treated with ondansetron, many patients managed with intravenous rehydration were admitted to hospital within hours of arriving to the PED, so that their stay in the PED was shorter. In addition, all patients who did not receive any form of treatment were within the group that did not receive ondansetron, so it is likely that patients with least severe disease, who would be discharged earlier, clustered in this group.
On the other hand, during data collection, we found heterogeneity in the use of ondansetron. Some clinicians administered ondansetron at the same time a catheter was inserted for delivery of intravenous fluids, thus eliminating one of the main potential benefits of the drug, which is avoiding intravenous rehydration. Similarly, in other cases the interval between the administration of ondansetron and initiation of intravenous rehydration was too short, which suggests that vomiting immediately after administration of the antiemetic was interpreted as a sign of treatment failure. However, since ondansetron is administered sublingually, it is not subject to the first-pass effect, and vomiting within a few minutes of its administration should not be interpreted as treatment failure.
Last of all, a recent meta-analysis18 attributed the heterogeneity of the results of studies published to date to baseline differences between the populations under study and differences in study design and methodology.
We ought to consider the limitations of our study. Among them are the limitations intrinsic to its retrospective design, as we were only able to obtain the data previously documented in the health records, which entails a loss of information. In addition, patients were not randomly assigned to treatment, so there may have been differences between the patients that comprised each group. In fact, the different distribution of patients aged less than 6 months compelled us to carry out a subanalysis of the results. Our study did not include all the patients with a diagnosis of gastritis/AGE managed in our PED in 2019 (but only 30.6%). Still, the sample was large (n = 825) and representative for the purpose of assessing real-world practice. In addition, some of the variables under study were subjective (such as the number of vomiting episodes or liquid stools and the degree of dehydration) and while other variables were not analysed, such as diarrhoea, which is one of the main complications of ondansetron. Lastly, there could be confounders at play, especially the age of the patient or disease severity. The above notwithstanding, our study was conducted in the context of real-world clinical practice, and provides an initial approximation to our clinical experience with ondansetron.
The conclusions of our study are:
- We found evidence of inappropriate use and abuse of ondansetron for management of vomiting associated with gastritis/AGE in the PED of our hospital. Of the total patients in our study, 38.8% received ondansetron when only 5.9% (19) had dehydration, which was mild in every case.
- Our findings suggest that ondansetron does not reduce the need of intravenous rehydration, especially in patients who are not dehydrated. However, it may be useful in patients with dehydration.
- Ondansetron seemed to reduce the rate of hospitalization, but it may not have the same effect once infants aged less than 6 months are excluded.
- Ondansetron does not seem to reduce the frequency of return visits to the PED within 72 hours.
- The length of stay in the PED may increase with the administration of ondansetron. However, other factors may be at play in this outcome, such as the severity of disease, the time when the patient is managed in the PED or the use of other treatments.
Therefore, the use of ondansetron for management of vomiting associated with gastritis/AGE in the PED of our hospital evinced abuse and inappropriate use of the drug. Our recommendations for appropriate use based on our clinical experience (medicine-based evidence) and the review of the scientific literature (evidence-based medicine) are: (1) ondansetron is not recommended for routine use in these patients; and (2) consider its use in paediatric patients (age > 6 months) with vomiting secondary to gastritis/AGE and with mild to moderate dehydration.
These recommendations are consistent with the consensus document recently published by the Working Group on Gastroenterology and Nutrition of the AEPap in terms of the favourable risk-benefit ratio of ondansetron for vomiting associated with AGE.28 At any rate, we consider that the main issue in the use of this drug is its appropriate prescribing, as the abuse of ondansetron in the PED evinced in our study may be reflected in a similar pattern at the primary care level.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article.
ABBREVIATIONS
AEPap: Asociación Española de Pediatría de Atención Primaria · AGE: acute gastroenteritis · CI: confidence interval · OR: odds ratio · PED: paediatric emergency department.
REFERENCES
- American Academy of Pediatrics. Practice parameter: the management of acute gastroenteritis in young children. Pediatrics. 1996;97:424-35.
- Guarino A, Ashkenazi S, Gendrel D, Lo Vecchio A, Shamir R, Szajewska H; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition; European Society for Pediatric Infectious Diseases. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59:132-52.
- Florez ID, Niño-Serna LF, Beltrán-Arroyave CP. Acute Infectious Diarrhea and Gastroenteritis in Children. Curr Infect Dis Rep. 2020;22:4.
- De la Torre Espí M, Molina Cabañero JC. Vómitos. In: Asociación Española de Pediatría [online] [accessed 09/04/2020]. Available at www.aeped.es/sites/default/files/documentos/vomitos_0.pdf
- Martín-Gavilán C, García-Avilés B, González-Montero R. Gastroenteritis aguda [Internet]. Alicante: Asociación Española de Pediatría [online] [accessed 09/04/2020]. Available at www.aeped.es/sites/default/files/documentos/gea.pdf
- Romano C, Dipasquale V, Scarpignato C. Antiemetic Drug Use in Children: What the Clinician Needs to Know. J Pediatr Gastroenterol Nutr. 2019;68:466-71.
- Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011;9:CD005506.
- DeCamp LR, Byerley JS, Doshi N, et al. Use of antiemetic agents in acute gastroenteritis: a systematic review and meta-analysis. Arch Pediatr Adolesc Med 2008;162:858-65.
- Metoclopramida: restricciones de uso en niños y adolescentes. Agencia española de medicamentos y productos sanitarios. In: Ministerio de Sanidad, Política Social e Igualdad. Madrid; 2011 [online] [accessed 08/04/2020]. Available at www.aemps.gob.es/informa/notasInformativas/medicamentosUsoHumano/seguridad/2011/docs/NI-MUH_20-2011.pdf?x38929
- Ficha técnica Zofran® 4 mg solución inyectable. Agencia española de medicamentos y productos sanitarios. Madrid; 2019 [online] [accessed 08/04/2020]. Available at https://cima.aemps.es/cima/dochtml/ft/59071/FT_59071.html
- Mosqueda Peña R, Rojo Conejo P. Gastroenteritis aguda. Asociación Española de Pediatría [online] [accessed 09/04/2020]. Available at https://www.aeped.es/sites/default/files/documentos/gastroenteritis_aguda.pdf
- Hagbom M, Novak D, Ekström M, Khalid Y, Andersson M, Lindh M, et al. Ondansetron treatment reduces rotavirus symptoms-A randomized double-blinded placebo-controlled trial. PLoS One. 2017;12:e0186824.
- Freedman SB, Pasichnyk D, Black KJL, Fitzpatrick E, Gouin S, Milne A, et al. Gastroenteritis therapies in developed countries: Systematic review and meta-analysis. PLoS One. 2015; 10:e0128754.
- Yilmaz HL, Yildizdas RD, Sertdemir Y. Clinical trial: oral ondansetron for reducing vomiting secondary to acute gastroenteritis in children - a double-blind randomized study. Aliment Pharmacol Ther. 2010;31:82-91.
- Marchetti F, Bonati M, Maestro A, Zanon D, Rovere F, Arrighini A, et al. Oral ondansetron versus domperidone for acute gastroenteritis in pediatric emergency departments: Multicenter double blind randomized controlled trial. PLoS One. 2016;11:e0165441.
- Ondansetrón. In: Asociación Española de Pediatría. Pediamécum [online] [accessed 09/04/2020]. Available at https://www.aeped.es/comite-medicamentos/pediamecum/ondansetron
- Freedman SB, Adler M, Seshadri R, et al. Oral ondansetron for gastroenteritis in a pediatric emergency department. N Engl J Med. 2006;354:1698-705.
- Wu HL, Zhan X. Effect of ondansetron on vomiting associated with acute gastroenteritis in a developing country: a meta-analysis. Eur J Pediatr. 2020;179:1181-9.
- Benary D, Lozano JM, Higley R, Lowe D. Ondansetron Prescription is associated with reduced return visits to the Pediatric Emergency Department for children with gastroenteritis. Ann Emerg Med. 2020;76:625-34.
- Freedman SB, Soofi SB, Willan AR, Williamson-Urquhart S, Ali N, Xie J, et al. Oral ondansetron administration to nondehydrated children with diarrhea and associated vomiting in Emergency Departments in Pakistan: a randomized controlled trial. Ann Emerg Med. 2019;73:255-65.
- Cheng A. Emergency department use of oral ondansetron for acute gastroenteritis-related vomiting in infants and children. Paediatr Child Health. 2011;16:177-82.
- Diarrhoea and vomiting caused by gastroenteritis in under 5s: diagnosis and management. In: National Institute for Health and Care Excellence. UK; 2019 [online] [accessed 15/04/2020]. Available at www.nice.org.uk/guidance/cg84/chapter/2-Research-recommendations#other-therapies-ondansetron
- Li ST, DiGiuseppe DL, Christakis DA. Antiemetic use for acute gastroenteritis in children. Arch Pediatr Adolesc Med. 2003;157:475-9.
- García de Paredes Esteban JC, Abdelkader Maanan M, Querol Gutiérrez JJ, Mohamed Haddu M, García Muñiz D. Protocolos conjunto de Atención Primaria-Atención Especializada para el manejo de medicamentos: Protocolo de uso racional de consansetrón. Madrid: Instituto Nacional de Gestión Sanitaria; 2020 [online] [accessed 26/05/2022]. Available at https://ingesa.sanidad.gob.es/bibliotecaPublicaciones/publicaciones/periodicasRevistas/docs/2020/ProtocolosC_V2_N2_2020.pdf
- Gorelick MH, Shaw KN, Murphy KO. Validity and reliability of clinical signs in the diagnosis of dehydration in children. 1997;99:E6.
- Freedman SB, Hall M, Shah SS, Kharbanda AB, Aronson PL, Florin TA, et al. Impact of increasing ondansetron use on clinical outcomes in children with gastroenteritis. JAMA Pediatr. 2014;168:321-9.
- Freedman SB, Soofi SB, Willan AR, Williamson-Urquhart S, Siddiqui E, Xie J, et al. Oral ondansetron administration to dehydrated children in Pakistan: a randomized clinical trial. Pediatrics. 2019;144:e20192161.
- Rodríguez Delgado J, Castell Miñana M, González Martín l, Hoyos Vázquez MS, Blesa Baviera LC; Grupo de Trabajo de Gastroenterología y Nutrición de la Asociación Española de Pediatría de Atención Primaria (AEPap). Uso de ondansetrón en el manejo de los vómitos asociados a gastroenteritis aguda en Pediatría de Atención Primaria. Posicionamiento del Grupo de Trabajo de Gastroenterología y Nutrición de la AEPap. Rev Pediatr Aten Primaria. 2021;23:e55-e64.





