Vol. 21 - Num. 82
Original Papers
Adenovirus infections that require hospital admission: epidemiology, laboratory findings and approach
Pedro Viaño Nogueiraa, Ana Moral Larraza, Irene Tomé Masaa, Marciano Sánchez Bayleb, Enrique Villalobos Pintoa, Marta Bascuas Arribasa, Francesco Ecclesiaa
aSección de Pediatría Hospitalaria. Hospital Infantil Universitario Niño Jesús. Madrid. España.
bPediatra. Fundación para la Investigación, Estudio y Desarrollo de la Salud Pública. Madrid. España.
Correspondence: P Viaño . E-mail: pedro.viano@salud.madrid.org
Reference of this article: Viaño Nogueira P, Moral Larraz A, Tomé Masa I, Sánchez Bayle M, Villalobos Pinto E, Bascuas Arribas M, et al. Adenovirus infections that require hospital admission: epidemiology, laboratory findings and approach. Rev Pediatr Aten Primaria. 2019;21:149-57.
Published in Internet: 13-06-2019 - Visits: 51631
Abstract
Introduction: adenovirus infections have a heterogeneous clinical presentation and are an important cause of childhood morbidity. They are frequently and unnecessarily treated with antibiotics. In this study, we analysed the characteristic of patients with adenovirus infections in order to determine whether they differed from those of patients with bacterial infection.
Patients and methods: the study included 174 patients admitted to a tertiary care hospital between January 2009 and August 2017 who tested positive for adenovirus. We analysed the clinical and laboratory findings in these patients and compared them to those of a group of patients that received a diagnosis of confirmed bacterial infection in the same hospital in 2016.
Results: the incidence of adenovirus was of 1.58 cases per 100 admissions. Sixty-four percent of the sample was male, and the mean age was 17 months. Patients that presented with gastrointestinal symptoms alone were younger and had more favourable laboratory findings compared to patients with respiratory symptoms alone. Coinfection with another virus was found in 24.5%, and this group had a longer length of stay (7.93 versus 6.17 days, p = 0.006). We found no significant differences in the laboratory criteria indicative of severe bacterial infection between the patients with adenovirus infection and the controls with a confirmed bacterial infection except for a very small, although statistically significant, difference in the levels of C-reactive protein.
Conclusions: the clinical and laboratory parameters analysed in our study are not sufficient to discriminate between bacterial infection and adenovirus infection. Thus, it would be appropriate to rule out adenovirus infection before initiating antibiotic treatment.
Keywords
● Acute phase proteins ● Adenovirus infections, human ● Coinfection ● Respiratory tract infectionsINTRODUCTION
Infection by adenovirus is a frequent cause of fever in children. Upper respiratory tract infections, such as pharyngitis or rhinitis, and lower respiratory tract infections, such as pneumonia, are the most frequent clinical presentations. Adenovirus infection can cause gastrointestinal, genital, urinary tract, ophthalmologic and neurologic illness less frequently.
Adenoviruses are a group of nonenveloped double-stranded DNA viruses. Seven species have been described this far (A-G). The species that are clinically relevant include B, C and E (associated with respiratory infection), D (which causes conjunctivitis) and F (associated with gastroenteritis).1
More than 80% of cases of confirmed adenovirus infection occur in children aged less than 4 years, probably due to the immaturity of their humoral immunity. Although most cases are self-limited, disseminated infection and pneumonia due to adenovirus may be life-threatening, especially in immunocompromised patients.
Outbreaks of adenovirus-related illness are most frequent in winter and spring. The virus may be transmitted by close contact (inhalation of saliva droplets, contact with conjunctival secretion or faecal-oral transmission) or through fomites. In addition, the virus may remain in lymphoid tissue or the renal parenchyma and undergo subsequent reactivation, a situation that is particularly likely in immunocompromised patients.
Acute respiratory tract infections are a significant cause of morbidity and mortality in early childhood worldwide. In addition, they are the most frequent form of infectious illness in the paediatric population in Spain.2-6
Although most cases are caused by viruses, there is an excessively frequent prescription of antibiotics, which are unnecessary in this type of infection. Their use does not achieve clinical improvement and may even be harmful, as it exposes patients to potential adverse events while increasing the prevalence of drug-resistant bacteria and thus decreasing the likelihood of an invasive bacterial infection responding to standard antibiotherapy.3,7-12
However, several studies have found that most physicians choose not to change the antibiotherapy regimen once a viral pathogen is detected in collected samples, partly due to the short age of the patients and also due to the customary practice of completing antibiotherapy regimens to decrease the likelihood of bacteria developing drug resistance. Nevertheless, ordering tests for detection of viral pathogens may be useful for the individual management of patients, to reduce the number of unnecessary diagnostic tests performed and to implement necessary isolation measures to decrease the incidence of nosocomial infection, thus reducing lengths of stay and the associated health care costs.5
The objectives of our study were to describe the clinical and laboratory characteristics associated with adenovirus infection, compare these variables in patients with coinfection by more than one viral pathogen, and analyse the differences between patients with adenovirus infection and patients with confirmed bacterial infections.
PATIENTS AND METHODS
Inclusion criteria
We included patients admitted to a tertiary care hospital in whom adenovirus was detected. The period under study ranged from January 2009 to August 2017 (a total of 8 years and 8 months). Tests for detection of adenovirus were ordered for all patients admitted with clinical manifestations suggestive of respiratory or gastrointestinal infection of unknown aetiology at admission.
We also analysed data for patients with a confirmed diagnosis of bacterial infection, which served as a control group for the purpose of comparing the laboratory characteristics associated with infection.
Detection methods
The assessment for adenovirus infection of the respiratory tract was made by collection a nasopharyngeal secretion aspirate sample either in the emergency department or in the first day of hospitalization followed by use of a commercial panel (Allplex Respiratory RP3, Seegene, Taewon Building, 91 Ogeum-ro, Songpa-gu, Seoul, South Korea) for detection of 19 respiratory viruses by means of real-time qualitative polymerase chain reaction: adenovirus, bocavirus, coronavirus NL63, 229E and OC43, enterovirus, influenza A (with specific differentiation of A-H1, A-H1pdm09 and A-H3) and B, metapneumovirus, parainfluenza 1 to 4, respiratory syncytial virus A and B and rhinovirus. Detection of adenovirus in the gastrointestinal tract consisted of collection of a sample of stool from a spontaneous bowel movement that was later subjected to a commercial assay for detection of adenovirus by real-time qualitative polymerase chain reaction.
Data collection
We collected data on the following variables: age, sex, date of admission, length of stay in days, duration of symptoms from onset to admission in days, number of siblings, presence of smokers in the household, history of illness in the household prior to admission, fever at admission, baseline oxygen saturation at admission, general health (good, fair, poor), counts/proportions of white blood cells (WBCs), neutrophils, band cells and platelets, serum levels of C-reactive protein (CRP) and procalcitonin (PCT), infection by other respiratory viruses, interpretation by radiologist of the chest radiograph (normal, abnormal), antibiotherapy and oxygen therapy during hospitalization.
Statistical analysis
We performed all the statistical analyses using the software IBM SPSS Statistics, version 15.0. We set the level of statistical significance at 0.05. We have expressed the results with as 95% confidence intervals.
We used the χ2 and the Fisher exact tests to compare qualitative variables. We used the Kolmogorov-Smirnov test to determine whether quantitative data followed a normal distribution and based on the results we used the Student t test to compare normally distributed variables and the Mann-Whitney U test otherwise.
To analyse diagnostic tests, we calculated odds ratios and the area under the curve (AUC) of receiver operating characteristics (ROC) curves.
RESULTS
During the period under study, there were a total of 174 admissions to the general paediatrics ward of our hospital of patients with infection by adenovirus out of a total of 10 990 admissions, which corresponds to an incidence of adenovirus infection of 1.58 cases per 100 admissions, with a high interannual variability (range, 0.41-2.18). When it came to the distribution by month of the year, we found that more than half of the cases were distributed between March (17.2%), April (12,6%), May (10.9%) and December (10.9%), while August was the month with the fewest admissions for this reason (1.7%). In our sample, 64.4% of patients were male and the mean age was 17 months. We found considerable variation in laboratory parameters, as a substantial number of patients had values suggestive of severe bacterial infection (WBC count >15 000 cells/µl, CRP >3 mg/dl, PCT >1 ng/ml), even though blood tests were not done in 24% of the patients. Table 1 presents the general characteristics of the overall sample.
| Table 1. Characteristics of patients with adenovirus | |
|---|---|
| Age (years), mean ± SD (minimum; maximum) | 1.39 ± 1.32 (0.8; 7.8) |
| Male, n (%) | 112 (64.4) |
| Fever at admission, n (%) | 119 (68.4) |
| Time from onset to admission (days), mean ± SD (minimum; maximum) | 4.16 ± 4.28 (0.1;30) |
| White blood cells ×1000/μl, mean ± SD (minimum; maximum) | 15.1 ± 8.24 (2.32; 56.4) |
| Neutrophils ×1000/μl, mean ± SD (minimum; maximum) | 8.93 ± 6.95 (0.39; 50.41) |
| C-reactive protein (mg/dl), mean ± SD (minimum; maximum) | 4.88 ± 5.32 (0.06; 29.3) |
| Procalcitonin (ng/ml) mean ± SD (minimum; maximum) | 1.65 ± 2.11 (0.1; 15) |
| White blood cells >15 000/μl, n (%) | 61 (35.1) |
| C-reactive protein >3 (mg/dl), n (%) | 63 (36.2) |
| Procalcitonin >1 (ng/ml), n (%) | 23 (13.2) |

Of all participants that answered the epidemiologic questions, 20 had a parent that smoked (11.5%) and 8 had a recent history of illness in the household (4.6%).
We found 54 cases presenting with gastrointestinal symptoms, associated with respiratory symptoms in 44.4%. Table 2 compares the characteristics of patients that presented only with gastrointestinal symptoms and those that presented only with respiratory symptoms. We found that patients with gastrointestinal symptoms alone were younger and had more favourable laboratory test results compared to patients with isolated respiratory symptoms.
| Table 2. Comparison of gastrointestinal infections and respiratory infections by adenovirus | |||
|---|---|---|---|
| GI | Respiratory | p-value | |
| Length of stay (days), mean ± SD | 6 ± 3 | 7 ± 4 | >0.05 |
| Age (years), mean ± SD | 0.77 ± 0.59 | 1.54 ± 1.50 | 0.006 |
| White blood cells ×1000/μl, mean ± SD | 13.16 ± 8.54 | 16.01 ± 8.83 | 0.036 |
| Neutrophils ×1000/μl, mean ± SD | 6.21 ± 4.91 | 9.94 ± 8.18 | 0.005 |
| C-reactive protein (mg/dl), mean ± SD(mg/dl) | 2.98 ± 4.83 | 5.26 ± 5.57 | 0.01 |
| Procalcitonin (ng/ml), mean ± SD | 1.01 ± 1.86 | 1.25 ± 2.47 | >0.05 |

A total of 92 (52.9%) patients received antibiotics in the emergency department or at some point during their hospital stay.
Nearly one-third of patients (51) had a viral coinfection: 11% by RSV, 8.2% by rhinovirus, 5.5% by rotavirus, 2.2% by bocavirus and 1% by influenzavirus.
Table 3 compares the characteristics of patients based on the presence or absence of coinfection. Patients with coinfection had longer lengths of stay, a difference that was statistically significant. They also tended to have a higher body temperature and increased elevation of laboratory parameters, with the exception of a WBC count above 15 000 cells/µl. Last of all, we ought to note that there was barely a difference in age between the two subsets of patients.
| Table 3. Comparison of patients with and without coinfection | |||
|---|---|---|---|
| Coinfection | No coinfection | p-value | |
| Length of stay (days), mean ± SD | 7.93 ± 4,45 | 6.17 ± 4,25 | <0.006 |
| Age (years), mean ± SD | 1.20 ± 1.32 | 1.45 ± 1.30 | >0.05 |
| Temperature (°C) | 37.61 ± 1.05 | 37.40 ± 1.04 | >0.05 |
| White blood cells × 1000/μl, mean ± SD | 16.18 ± 10.03 | 14.51 ± 7.28 | >0.05 |
| Neutrophils ×1000/μl, mean ± SD | 9.37 ± 9.17 | 8.67 ± 5.76 | >0.05 |
| C-reactive protein (mg/dl), mean ± SD | 5.92 ± 6.12 | 4.48 ± 5.03 | >0.05 |
| Procalcitonin (ng/ml), mean ± SD | 1.27 ± 1.78 | 1.14 ± 2.31 | >0.05 |
| White blood cells >15 000, n (%) | 16 (40) | 45 (48.9) | >0.05 |
| C-reactive protein >3 (mg/dl), n (%) | 21 (58.3) | 43 (47.2) | >0.05 |
| Procalcitonin >1 (ng/ml), n (%) | 10 (34.4) | 14 (24.1) | >0.05 |
| Platelets >400 000/μl, n (%) | 22 (51.1) | 46 (35.3) | >0.05 |
| Antibiotherapy, n (%) | 26 (59) | 66 (48.5) | >0.05 |
| ICU admission, n (%) | 5 (11.1) | 11 (8.2) | >0.05 |
| Positive blood culture, n (%) | 2 (4.6) | 1 (0.7) | >0.05 |
| Oxygen therapy, n (%) | 23 (56.1) | 64 (50) | >0.05 |

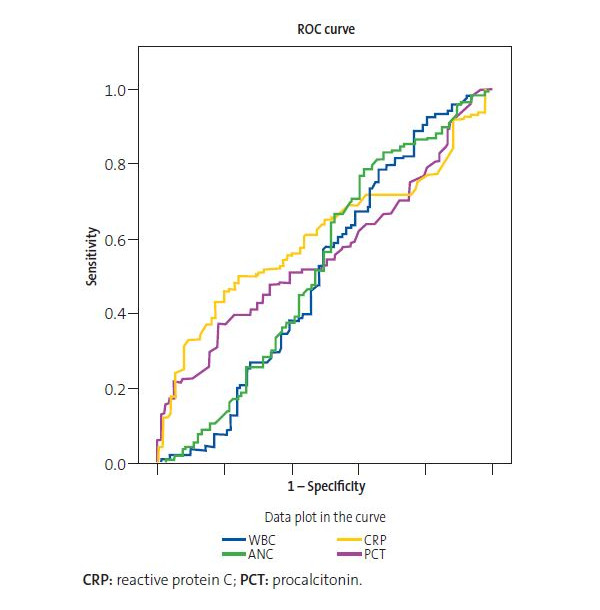
When we compared the selected patients with adenovirus with the controls that had a bacterial infection confirmed by culture, we found no significant differences in the 3 laboratory criteria suggestive of severe bacterial infection (WBC count >15 000 cells/μl, CRP >3 mg/dl, PCT >1 ng/ml), as can be seen in Table 4. These findings were corroborated by the ROC curve shown in Figure 1. Our findings demonstrated that laboratory parameters were of little use to distinguish between bacterial infections and adenovirus infection. As can be seen in Table 5, the only AUC that was statistically significant was the one corresponding to the CPR level, although it showed that it had a negligible power to discriminate between bacterial and viral infection.
| Table 4. Comparison of patients with bacterial infection and adenovirus infection | ||||
|---|---|---|---|---|
| Bacterial infection | Adenovirus | OR | IC | |
| At least 1 criterion | 92 | 62 | 1.30 | (0.66-2.77) |
| At least 2 criteria | 58 | 33 | 1.54 | (0.84-2,84) |
| All 3 criteria | 23 | 12 | 1.48 | (0.65-3.41) |

| Figure 1. Receiver operating characteristic (ROC) curve of laboratory parameters used to compare bacterial infection and adenovirus infection |
|---|
 |

| Table 5. Area under the curve of laboratory parameters used to compare bacterial infection and adenovirus infection | ||||
|---|---|---|---|---|
| Outcome variables | AUC | p | 95% confidence interval | |
| Upper limit | Lower limit | |||
| White blood cells | 0.508 | 0.850 | 0.423 | 0.593 |
| Total neutrophils | 0.527 | 0.514 | 0.442 | 0.612 |
| C-reactive protein | 0.609 | 0.009 | 0.531 | 0.687 |
| Procalcitonin | 0.559 | 0.159 | 0.479 | 0.638 |

DISCUSSION
The incidence of adenovirus infection in our study was of 1.58/100 admissions to the General Paediatrics Ward of our hospital, with a wide annual variability that was most likely the result of detection bias.
Spring was the season in which there were the most admissions due to adenovirus infection. Of all patients with this diagnosis, 64.4% were male. Both findings were consistent with the existing medical literature.5
There was coinfection with another virus in 24.5% of the patients, a proportion that was similar to those reported by other authors.5,13 In the subset of patients with coinfection, 11% had infection by respiratory syncytial virus (RSV) and 8.2% by rhinovirus. These 2 viruses are the viruses detected most frequently in patients with acute upper respiratory tract infections in most of the studies we reviewed, which would explain why they were the viruses found most frequently in association with adenovirus in our study.5
As for the age of patients, we found significant differences based on the presence or absence of coinfection, as the latter was more frequent with decreasing age. This was consistent with the findings of previous studies, where the authors reported a lower rate of coinfection in older children5,14; this could be attributed to a lower clearance of the virus in younger children due to the immaturity of their immunity against viral pathogens.5
When it came to the need for oxygen therapy, we did not find significant differences between patients with viral coinfection and patients without, which was similar to the findings of most previous studies we reviewed, although some authors found that coinfection was associated with a lower frequency of oxygen therapy.5,13
In addition, the length of stay was longer in patients with coinfection by other respiratory viruses compared to patients with isolated infection by adenovirus. This finding diverged from the findings of other studies where patients with infection by a single pathogen had a longer mean length of stay compared to patients with coinfection by other respiratory viruses.5
Different authors have presented contradictory results on the association between coinfection and the severity of acute illness. Some have found that illness is more severe if the infection involved several viral pathogens,15-21 while others did not find any differences in severity between cases with and without infection by multiple viruses.18,22-31 There are even studies that have reported that patients with viral coinfection required admission to intensive care units less frequently compared to patients infected by a single virus, which could be due to undetected viral-bacterial coinfections, which evinces that in the future it would be advisable to research the different types of coinfection present in these patients.5,13,30,32-37
In regard to the laboratory parameters, CPR is an acute-phase protein indicative of tissue damage due processes such as infection, trauma or inflammation, so it is essential to take into account the clinical context of the patient in the interpretation of its values and to remember that it cannot effectively discriminate between viral and bacterial infection,3 although some authors have found that a serum CPR greater than 40 to 60 mg/l was significantly more frequent in patients with bacterial infection.38
In our sample, CRP levels above 3 mg/dL were more frequent in the coinfection group (58.3%) compared to the isolated adenovirus infection group (47.2%). We also found a higher mean CPR level in the former group. In both cases, the difference was not statistically significant.
The comparison of some of the findings of blood tests suggestive of severe bacterial infection in the patients included in our sample to those in patients with a confirmed bacterial infection (WBC count >15 000 cells/µL, CRP > 3 mg/dL, PCT > 1 ng/mL) revealed no significant differences, which illustrates the limited usefulness of blood tests when it comes to differentiating between a viral and a bacterial aetiology, which was further corroborated by the AUC found for the laboratory parameters included in our analysis, which did not exceed 0.609 in any case (the value for the CPR AUC, which was the highest). Some authors have noted that viral respiratory tract infections increase susceptibility to bacterial infection due to their effects on physical barriers and the immune system.39
The main limitations of our study are those intrinsic to retrospective studies and that since the sample was exclusively of inpatients, we did not include any data on cases managed at the outpatient level. In addition, there may be a mild elevation of CRP after infection has resolved,5,14,40 although bacteriological tests were negative in all such cases.
In conclusion, adenovirus infection is a frequent cause of hospital admission in the paediatric age group, especially in male individuals and in the spring. It is frequently associated to infection by other viruses, which increases the length of stay and serum CPR levels. The laboratory parameters that are usually indicative of infection do not allow differentiation between a viral and a bacterial aetiology, so it would be reasonable to rule out infection by adenovirus prior to initiation of antibiotherapy. Further studies are required to better define the characteristics and associated factors of adenovirus infection in paediatric patients.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article.
ABBREVIATIONS
CPR: C-reactive protein · PCT: procalcitonin · RSV: respiratory syncytial virus.
REFERENCES
- Lynch JP, Kajon AE. Adenovirus: epidemiology, global spread of novel serotypes, and advances in treatment and prevention. Semin Respir Crit Care Med. 2016;37:586-602.
- Marcone DN, Durand LO, Azziz-Baumgartner E, Vidaurreta S, Ekstrom J, Carballal G, et al. Incidence of viral respiratory infections in a prospective cohort of outpatient and hospitalized children aged ≤5 years and its associated cost in Buenos Aires, Argentina. BMC Infect Dis. 2015;15:447.
- Llor C, Alkorta M, de la Flor J, Bernárdez S, Cañada JL, Bárcena M, et al. Recomendaciones de utilización de técnicas de diagnóstico rápido en infecciones respiratorias en Atención Primaria. Aten Primaria. 2017;49:426-37.
- Jevsnik M, Ursic T, Zigon N, Lusa L, Krivec U, Petrovec M. Coronavirus infections in hospitalized pediatric patients with acute respiratory tract disease. BMJ Infect Dis. 2012;12:365.
- Huijskens EG, Biesmans RC, Buiting AG, Obihara CC, Rossen JW. Diagnostic value of respiratory virus detection in symptomatic children using real-time PCR. Virol J. 2012;9:276.
- Kahn JS. Newly discovered respiratory viruses: significance and implications. Curr Opin Pharmacol. 2007;7:478-83.
- Rosenstein N, Phillips WR, Gerber MA, Marcy SM, Schwartz B, Dowell SF. The common cold-Principles of judicious use of antimicrobial agents. Pediatrics. 1998;101:181-4.
- Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735-43.
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
- Dowell SF, Schwartz B. Resistant pneumococci: protecting patients through judicious antibiotic use. Am Fam Physician. 1997;55:1647-54.
- Van de Pol AC, Wolfs TF, Jansen NJ, van Loon AM, Rossen JW: Diagnostic value of real-time polymerase chain reaction to detect viruses in young children admitted to the paediatric intensive care unit with lower respiratory tract infection. Crit Care. 2006;10:R61.
- Wishaupt JO, Russcher A, Smeets LC, Versteegh FG, Hartwig NG. Clinical impact of RT-PCR for pediatric acute respiratory infections: a controlled clinical trial. Pediatrics. 2011;128:e1113-20.
- Chorazy ML, Lebeck MG, McCarthy TA, Richter SS, Torner JC, Gray GC. Polymicrobial acute respiratory infections in a hospital-based pediatric population. Pediatr Infect Dis J. 2013;32:460-6.
- Cebey-López M, Herberg J, Pardo-Seco J, Gómez-Carballa A, Martinón-Torres N, Salas A, et al. Viral co-infections in pediatric patients hospitalized with lower tract acute respiratory infections. PLoS One. 2015;10:e0136526.
- Bharaj P, Sullender WM, Kabra SK, Mani K, Cherian J, Tyagi V, et al. Respiratory viral infections detected by multiplex PCR among pediatric patients with lower respiratory tract infections seen at an urban hospital in Delhi from 2005 to 2007. Virol J. 2009;6:89.
- Bonzel L, Tenenbaum T, Schroten H, Schildgen O, Schweitzer-Krantz S, Adams O. Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real-time polymerase chain reaction. Pediatr Infect Dis J. 2008;27:589-94.
- Jennings LC, Anderson TP, Beynon KA, Chua A, Laing RT, Werno AM, et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63:42-8.
- Midulla F, Scagnolari C, Bonci E, Pierangeli A, Antonelli G, De Angelis D, et al. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Child. 2010;95:35-41.
- Templeton KE, Scheltinga SA, van den Eeden WC, Graffelman AW, van den Broek PJ, Claas EC. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41:345-51.
- Yoshida LM, Suzuki M, Yamamoto T, Nguyen HA, Nguyen CD, Nguyen AT, et al. Viral pathogens associated with acute respiratory infections in central Vietnamese children. Pediatr Infect Dis J. 2010;29:75-7.
- Paranhos-Baccalà G, Komurian-Pradel F, Richard N, Vernet G, Lina B, Floret D. Mixed respiratory virus infections. J Clin Virol. 2008;43:407-10.
- Gil J, Almeida S, Constant C, Pinto S, Barreto R, Cristino JM, et al. Relevancia a corto plazo de la coinfección viral en pacientes menores de 2 años hospitalizados con infecciones de las vías respiratorias inferiores. An Pediatr (Barc). 2018;88:127-35.
- Reina J, Ferrés F, Rubio R, Rojo-Molinero E. Análisis de las coinfecciones detectadas entre los subtipos del virus respiratorio sincitial y otros virus respiratorios. An Pediatr (Barc). 2015;82:e255-6.
- Harada Y, Kinoshita F, Yoshida LM, Minh LN, Suzuki M, Morimoto K, et al. Does respiratory virus coinfection increases the clinical severity of acute respiratory infection among children infected with respiratory syncytial virus? Pediatr Infect Dis J. 2013;32:441-5.
- Suryadevara M, Cummings E, Bonville CA, Bartholoma N, Riddell S, Kiska D, et al. Viral etiology of acute febrile respiratory illnesses in hospitalized children younger than 24 months. Clin Pediatr (Phila). 2011;50:513-7.
- Brand HK, de Groot R, Galama JM, Brouwer ML, Teuwen K, Hermans PW, et al. Infection with multiple viruses is not associated with increased disease severity in children with bronchiolitis. Pediatr Pulmonol. 2012;47:393-400.
- Peng D, Zhao D, Liu J, Wang X, Yang K, Xicheng H, et al. Multipathogen infections in hospitalized children with acute respiratory infections. Virol J. 2009;6:155.
- Brouard J, Freymuth F, Vabret A, Jokic M, Guillois B, Duhamel JF. Co-infections virales lors des bronchiolites du nourrisson immunocompetent: etude prospective epidemiologique. Arch Pediatr. 2000;7 Suppl 3:S531-5.
- Drews AL, Atmar RL, Glezen WP, Baxter BD, Piedra PA, Greenberg SB. Dual respiratory virus infections. Clin Infect Dis. 1997;25:1421-9.
- Fabbiani M, Terrosi C, Martorelli B, Valentini M, Bernini L, Cellesi C, et al. Epidemiological and clinical study of viral respiratory tract infections in children from Italy. J Med Virol. 2009;81:750-6.
- Jennings LC, Anderson TP, Beynon KA, Chua A, Laing RT, Werno AM, et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63:42-8.
- Van der Zalm MM, van Ewijk BE, Wilbrink B, Uiterwaal CS, Wolfs TF, van der Ent CK. Respiratory pathogens in children with and without respiratory symptoms. J Pediatr. 2009;154:396-400, 400 e1.
- Aberle JH, Aberle SW, Pracher E, Hutter HP, Kundi M, Popow-Kraupp T. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon-gamma response. Pediatr Infect Dis J. 2005;24:605-10.
- Choi EH, Lee HJ, Kim SJ, Eun BW, Kim NH, Lee JA, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005. Clin Infect Dis. 2006;43:585-92.
- Huang JJ, Huang TY, Huang MY, Chen BH, Lin KH, Jeng JE, et al. Simultaneous multiple viral infections in childhood acute lower respiratory tract infections in southern Taiwan. J Trop Pediatr. 1998;44:308-11.
- Legg JP, Warner JA, Johnston SL, Warner JO. Frequency of detection of picornaviruses and seven other respiratory pathogens in infants. Pediatr Infect Dis J. 2005;24:611-6.
- Peng D, Zhao D, Liu J, Wang X, Yang K, Xicheng H, et al. Multipathogen infections in hospitalized children with acute respiratory infections. Virol J. 2009;6:155.
- Flood RG, Badik J, Aronoff SC. The utility of serum C-reactive protein in differentiating bacterial from nonbacterial pneumonia in children: a meta-analysis of 1230 children. Pediatr Infect Dis J. 2008;27:95-9.
- Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23:74-98.
- Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72:695-9.





