Vol. 17 - Num. 65
Original Papers
Bacterial gastroenteritis in a Zaragoza health care area (Spain)
Elena Laín Mirandaa, Sonia Ruiz Aliendea, Carmen Marne Traperob, María José Revillo Pinillab
aMIR-Microbiología. Hospital Universitario Miguel Servet. Zaragoza. España.
bServicio de Microbiología. Hospital Universitario Miguel Servet. Zaragoza. España.
Correspondence: E Laín. E-mail: melainm@salud.aragon.es
Reference of this article: Laín Miranda E, Ruiz Aliende S, Marne Trapero C, Revillo Pinilla MJ. Bacterial gastroenteritis in a Zaragoza health care area (Spain). Rev Pediatr Aten Primaria. 2015;17:29-35.
Published in Internet: 10-03-2015 - Visits: 23073
Abstract
Objective: acute gastrointestinal infections are one of the most frequent infectious diseases in primary health care. Implication of microorganisms is variable in different geographical areas, according to seasonal period and population studied. The aim is to study retrospectively the stool cultures and the Clostridium difficile toxin A/B performed from 2010 to 2012 in the Hospital Miguel Servet (Zaragoza, Spain) of paediatric patients from the area II of Zaragoza.
Materials and methods: 24058 faeces were received for culture (46.6% from children) and 4132 faeces for toxin investigation (3.4%from children).
Results: 9.6% of the stool cultures were positive for one or more enteropathogen bacteria (14.8% from adults and 5.1% from children). Five percent of the toxin investigations were positive (8.6% from children and 4.9 % from adults).
Conclusions: the bacteria most frequently isolated in both adults and children were Campylobacter (49.9% of the positives in children and 37.1% in adults) and Salmonella (33.8% of the positives in children and 32.9% in adults). The most frequent serotype of Salmonella was Salmonella enteritidis and the most frequent species of Campylobacter was Campylobacter jejuni.
Keywords
● Diarrhoea ● Enteropathogen ● GastroenteritisINTRODUCTION
Infections of the gastrointestinal tract, along with respiratory tract and genital and urinary tract infections, constitute some of the most frequent infectious diseases seen in Primary Care. While they are often a benign entity in healthy adults, a water-electrolyte imbalance can cause dehydration in children, the elderly and at-risk individuals. Gastrointestinal infections continue to be one of the leading causes of morbidity and mortality in infants worldwide.1
The involvement of different microorganisms varies across geographical areas, and also depends on the segment of the population under study. Bacteria are the second leading causative agent of gastroenteritis, following viruses, while parasites are the least frequent aetiology. When it comes to seasonal predominance, there is a higher incidence of viral gastroenteritis during autumn and winter, while bacterial meningitis is most frequent in the spring and summer.2
The main symptoms of gastroenteritis are diarrhoea, fever, vomiting and abdominal pain. Gastroenteritis can be classified depending on the type of diarrhoea into secretory gastroenteritis (watery) or dysentery (invasive). Watery or secretory diarrhoea is more frequent and usually manifests with watery stools. In invasive diarrhoea or dysentery, the stools may contain blood, mucus or pus, and the diarrhoea may be accompanied by fever, abdominal pain and tenesmus. Laboratory tests are needed to reach a definitive diagnosis.
The aim of this study was to learn the results of stool cultures and of Clostridium difficile toxin A/B tests performed in the paediatric population of health care area II in Zaragoza (Spain).
MATERIALS AND METHODS
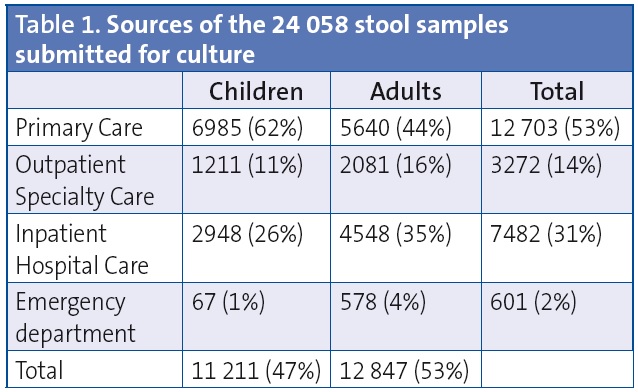
The Microbiology Department of the Hospital Universitario Miguel Servet of Zaragoza received 24 058 samples for stool culture from January 2010 through December 2012, of which 46.6% corresponded to patients in the paediatric age group. The distribution by sex was fairly even. Most of the stool samples were from Primary Care patients (Table 1).
The stool samples were received in a sterile container without transport medium and were seeded directly on the culture media generally used for the isolation of the most frequent causative agents of diarrhoea, including:
- Blood agar for a general overview of the current intestinal flora.
- XLD (xylose, lysine, deoxycholate) agar for isolation of Salmonella and Shigella.
- CIN (cefsulodin, irgasan, novobiocin) agar for isolation of Yersinia and Aeromonas.
- CCD (charcoal, cefoperazone, deoxycholate, amphotericin) agar for isolation of Campylobacter.
- For watery stools, a SMAC (sorbitol-McConkey) agar was added for isolation of enterohaemorrhagic Escherichia coli O157.
- The samples were also seeded in tetrathionate broth for selective enrichment of Salmonella, and reseeded on Hektoen enteric agar 24 hours later.
The blood agar, XLD, SMAC and Hektoen plates were incubated aerobically at 35 °C for 24 hours, the CIN plate aerobically at 30 °C for 24 hours, and the CCD plate at 42 °C in a microaerophilic atmosphere and read at 24 and 48 hours.
Microorganisms were identified by MALDI-TOF® mass spectrometry (Brucker Daltonik). The identification of Salmonella, Shigella and Yersinia isolates was completed by agglutination tests with commercial antisera. The Salmonella strains were sent to the Centro Nacional de Microbiología (National Centre of Microbiology) for epidemiological serotyping and phage-typing testing. Antibiotic susceptibility testing was performed by broth microdilution (MicroScan Walkaway®, Siemens) and in the case of Campylobacter by the disk diffusion method on blood agar.
The department received 4132 stool samples for Clostridium difficile testing, 3.41% of which came from Paediatrics. Clostridium difficile toxin A/B detection was performed by means of microplate immunoassay, and positive results were confirmed by cell cytotoxicity assays.
RESULTS
Of the 24 058 stool cultures performed, 9.6% were positive for one or more enteropathogens. Positive results were found in 14.8% of the 11 211 paediatric samples and 5.1% of the 12 847 samples from adult patients.
Table 2 details the microbiological results obtained in paediatric patients compared to adult patients. The bacteria isolated most frequently both in children and adults were Campylobacter (49.9% of positive tests in children and 37.1% in adults) and Salmonella (33.8% of positive tests in children and 32.9% in adults). The serotype that predominated in the broad antigenic spectrum of Salmonella corresponded to the typhimurium serovar. The second most frequent genus was Aeromonas. The most frequently isolated species in the Campylobacter genus was C. jejuni (Table 2). Of the 4132 samples tested for C. difficile toxins A/B, 5% tested positive (8.6% of the 128 paediatric samples and 4.9% of the 4004 adult samples) (Table 2).
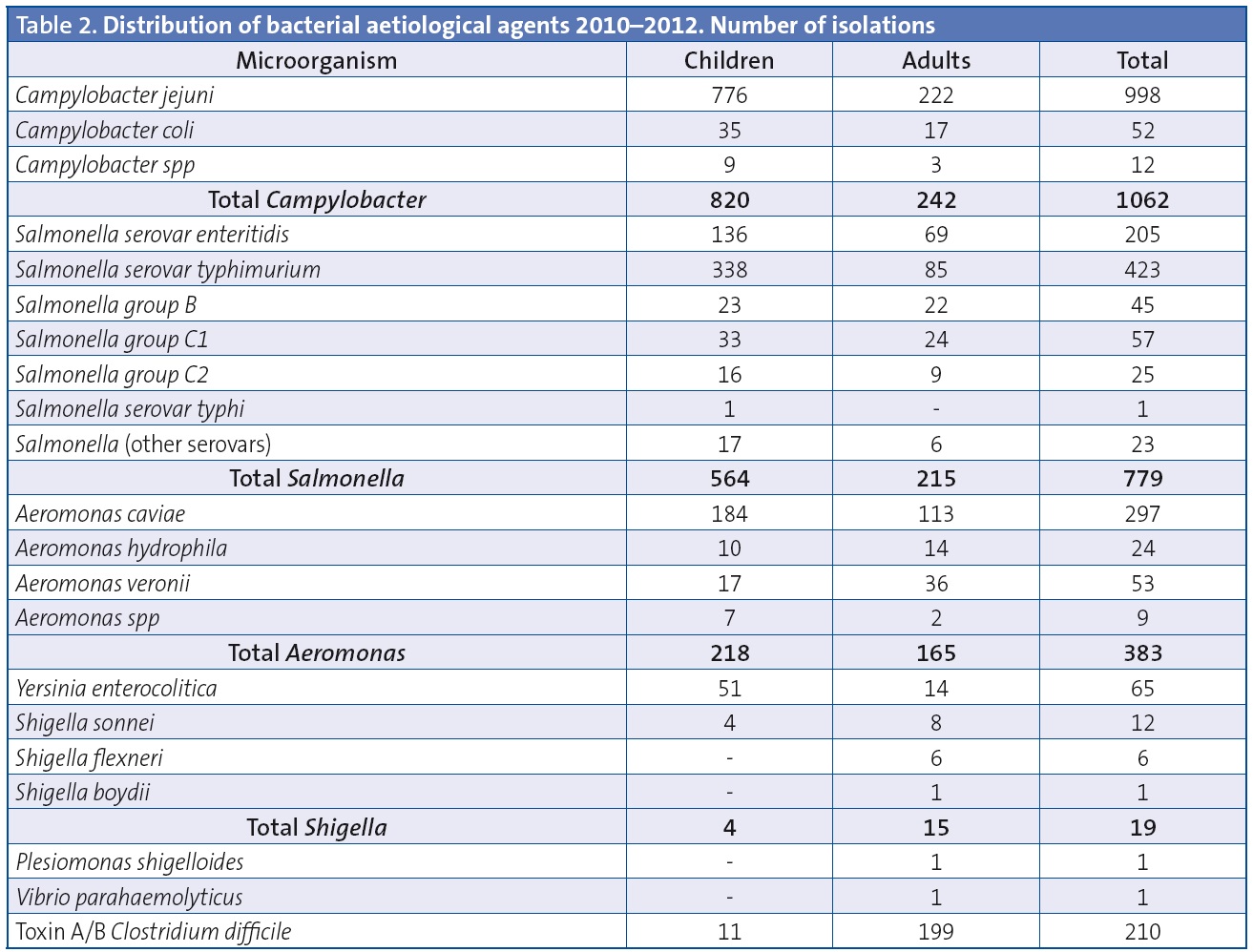
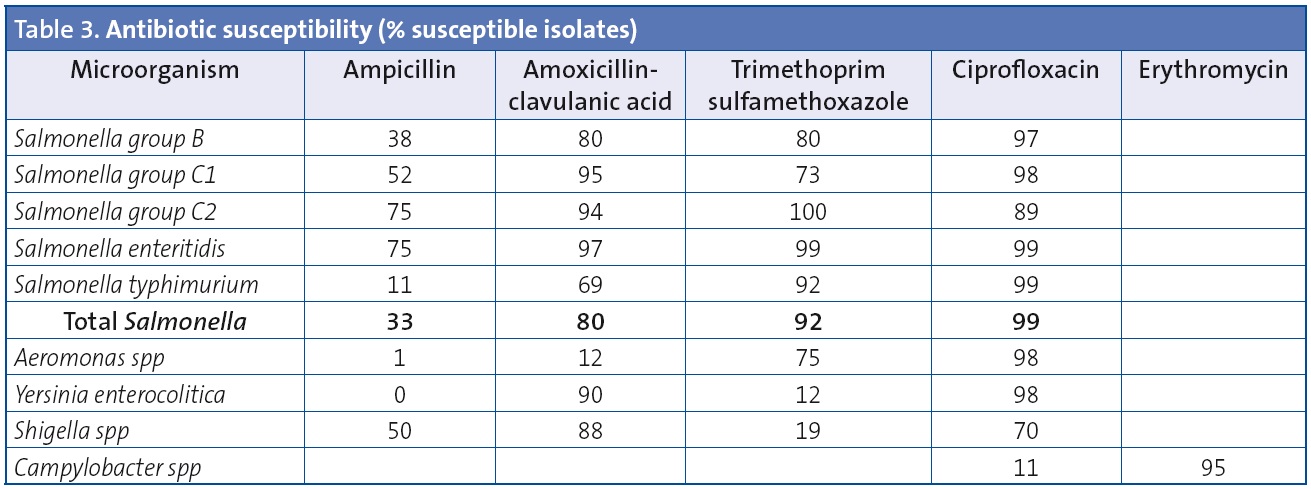
Table 3 shows the results of antibiotic susceptibility testing for the main enteropathogenic bacteria isolated from stool cultures.
DISCUSSION
The bacteriological study of diarrhoeic illnesses is a significant component of the work in a microbiology laboratory, both in terms of the cost of the materials used and the time invested. However, there is generally a low yield of positive results with isolation and identification of the bacterial aetiological agent. There are many reasons for this. The lack of demographic and clinical data for the patient and of an orientation for the diagnosis in the order form prevents the selection of appropriate methods due to the broad spectrum of agents that may be involved (bacteria, viruses, toxins, parasites). The age, symptoms, type of diarrhoea, whether the case is isolated or part of an outbreak, travel to endemic countries and any other data of interest can facilitate the microbiological diagnosis.
Enteropathogenic bacteria are very labile to stool changes in pH, temperature and humidity, so samples ought to be taken soon after the onset of diarrhoea and transported and processed in a timely manner. The submission of multiple samples increases the workload of the laboratory and does not substantially improve the yield, as initial samples are the best. Submission of samples from patients with clinical improvement is not recommended due to the high probability of obtaining a false-negative result. In our laboratory, 28% of the stools received were inadequate because they were formed stools with a solid consistency.
The bacteria most frequently isolated in our study in both children and adults were Campylobacter and Salmonella, which was consistent with the reviewed medical literature.2,3 The increase in exotic pet species in the homes of children, especially of cold-blooded animals, has resulted in the sporadic appearance of atypical Salmonella serotypes in our country.4 The Campylobacter species isolated most frequently was C. jejuni, consistent with previous studies.5From an epidemiological standpoint, Shigella and Yersinia are not a public health concern due to their low incidence.
The pathogenic role of Aeromonas in cases of diarrhoea is controversial, as it has not been possible to reproduce it in animal models, although A. hydrophila and A. veroni are potentially invasive species. Their isolation from stools may reflect transient colonisation. In developed countries, the Aeromonas carriage rate ranges between 2% and 10%.7 Most of the summer increase in the isolation of Aeromonas without a clear pathogenic role in diarrhoeic illness is probably explained by the greater growth rate of this bacterium in foods and recreational water environments promoted by higher environmental temperatures. Usually, these are mild self-limited illnesses associated with recent consumption of fish or shellfish. Its presence in cases of subacute diarrhoea (from two weeks to two months) or chronic diarrhoea (more than two months) may be underestimated. While the available data are not conclusive, Aeromonas may play an important role in traveller’s diarrhoea. It may occasionally manifest as watery secretory diarrhoea, and in very severe cases it has been associated with haemolytic uraemic syndrome.7
Recently, testing for C. difficile toxins has gained relevance. It used to be performed only in hospitalised patients, but it must be considered in specific Primary Care cases, always adhering to the guidelines established with the laboratory. It is not recommended for ages less than 2 years due to the difficulty in interpreting the results, as carriage is very common in this age group.6 While the total number of C. difficile toxin A/B detections was not high, 8.6% of paediatric tests and 4.9% of adult tests were positive. The implementation of protocols will help establish the actual incidence of this disease in Primary Care.6
The primary treatment of bacterial infectious enteritis is oral rehydration with early introduction of usual foods, with the diarrhoea resolving within a few days. Breastfeeding should not be discontinued.8,9 Admission to the hospital is only warranted when the patient has poor oral tolerance or is dehydrated. Pharmacological treatment, and especially antibiotic therapy, is not recommended for the systematic treatment of all cases. The type of diarrhoea, clinical status, and involved pathogen must be taken into account. In gastroenteritis cases caused by Shigella and of dysentery caused by Campylobacter and Salmonella, early treatment reduces the symptoms, the risk of bacteraemia and the risk of transmission by shortening the duration of bacterial shedding in the stool.8 In cases of watery diarrhoea caused by Salmonella, signs of initial improvement with treatment may be deceptive, as cultures become negative in early stages but the rate of positive results increases at a later point. Treatment can lead to a higher relapse rate, cause adverse effects and increase bacterial resistance.8,10 Treatment is indicated in newborns and immunocompromised patients. Follow-up stool cultures are not recommended in cases of self-limiting disease in immunocompetent patients.11
Some of the antibiotics used most frequently in the treatment of Salmonella infections are amoxicillin-clavulanic acid, fluoroquinolones (in adults or in older children when there is no other choice) and trimethoprim-sulfamethoxazole.12-17 The overall rate of ampicillin resistance of the Salmonella isolates in this study was 77%. The higher rates of resistance were found in the isolates of serovar typhimurium (88%) compared to serovar enteritidis (25%). The rate of resistance to amoxicillin-clavulanic acid was 20%. The overall susceptibility to ciprofloxacin for all serotypes was approximately 100%. These antibiotic susceptibility results are similar to those found in other studies conducted in Spain.14-17
Campylobacter resistance to fluoroquinolones has increased sharply in various countries since the 1990s, and has been recognised as a public health problem. This increase has coincided with the approval of fluoroquinolones for veterinarian use. In our study, 89% of the isolates were resistant to ciprofloxacin and 5% to erythromycin, figures that are similar to those reported by other authors. While the rate of macrolide resistance in clinical isolates of Campylobacter is not alarming, an increase in this rate has been documented both in animals and in humans.18
The most effective prevention strategies are health education, strict hygiene measures to prevent person-to-person transmission, and in the case of Salmonella, treatment of carriers. Chronic carriage is defined as detection of Salmonella in stool or urine samples over a period of more than one year.10 Eradication of chronic carriage is only indicated in healthcare workers, nursing home staff, patients with human immunodeficiency virus (HIV) infection, food handlers and members of the household of an immunocompromised individual.11
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article.
ABBREVIATIONS: CCD, charcoal, cefoperazone, deoxycholate, amphotericin; CIN: cefsulodin, irgasan, novobiocin; HIV: human immunodeficiency virus; SMAC, sorbitol-McConkey agar; XLD, xylose, lysine, deoxycholate.
REFERENCES
- Vila J, Álvarez-Martínez MJ, Buesa J, Castillo J. Diagnóstico microbiológico de las infecciones gastrointestinales. Enferm Infecc Microbiol Clin. 2009;27:406-11.
- Álvarez Martínez M, Buesa Gómez J, Castillo García J, Vila Estape J. Diagnóstico microbiológico de las infecciones gastrointestinales. Procedimientos en Microbiología Clínica. En: Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica [en línea] [consultado el 27/02/2015]. Disponible enwww.seimc.org/
- Fischer Walker CL, Sack D, Black RE. Etiology of diarrhea in older children, adolescents and adults: a systematic review. PloS Negl Trop Dis. 2010;4:e768.
- Palomar Saiz S, Lopes Semedo G, Montejo Martínez MC, Cortés Rico O, María Tablado MA. Abordaje de una microepidemia de salmonelosis en el seno de una familia. Rev Pediatr Aten Primaria. 2011;20:e14.
- Van Trieu T, De Pontual L. Conduite à tenir devant une diarrhée aigüe chez l’enfant. Presse Med. 2013;42:60-5.
- Bouza E, Marín M, Peláez T, Group for Clostridium difficile infection of the Spanish Society for Chemotherapy. The situation and management of Clostridium difficile infection in Spain: an opinion document. Rev Esp Quimioter. 2013;26:261-86.
- Janda MJ, Abbott SL. The genus Aeromonas: raxonomy, pathogenicity and infection. Clin Microbiol Rev. 2010;23:35-73.
- Costa i Pagès J, Polanco Allué I, Gonzalo de Liria CR. Guía de Práctica Clínica. Gastroenteritis aguda en el niño. Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica [en línea] [consultado el 27/02/2015]. Disponible en www.guiasalud.es
- Gutiérrez Castrellón P, Polanco Allue I, E. Salazar Lindo E. Manejo de la gastroenteritis aguda en menores de 5 años: un enfoque basado en la evidencia. Guía de práctica clínica Íbero-Latinoamericana. An Pediatr (Barc). 2010;72:220.e1-220.e20.
- Colaboración Cochrane. Antibióticos en infecciones intestinales por salmonela. Rev Pediatr Aten Primaria. 1999;1:453-6.
- Pegues DA, Miller SI. Salmonella species, including Salmonellatyphi. En: Mandell GL, Bennet JE, Dolin R (eds). Mandell, Douglas and Bennett’s principles and practice of infectious diseases. 7.ª edición. Filadelfia (PA): Churchill Livingstone Elsevier; 2010. p. 2887-2903.
- Mensa J, Gatell JM, García-Sánchez JE, Letang E, López-Suñé E, Marco F. Guía de terapéutica antimicrobiana. 23.ª edición. Barcelona: Antares; 2014.
- Gendrel D, Cohen R. Diarrhées bactériennes et antibiotiques: les recommandations européenes. Arch Pediatr. 2008;15:S93-6.
- Delgado Ronda N, Muñoz Bellido JL, Ibáñez Pérez R, García García MI, Serrano Heranz R, Muñoz Criado S, et al. Resistencia a antimicrobianos en Salmonella no typhi en Castilla y León. Rev Esp Quimioterap. 2004;1729-36.
- Guerri Santos ML, Rotger R. Evolución de la resistencia a quinolonas y betalactámicos en distintos serogrupos de Salmonella durante la última década en un centro hospitalario de Madrid. Rev Esp Quimioter. 2004;17:37-43.
- Onwuezobe IA, Oshun O, Odigwe CC. Antimicrobials for treating symptomatic non-typhoidal Salmonella infection. Cochrane Database Syst Rev. 2012 Nov 14;11:CD001167.
- De Toro M, Seral C, Rojo-Bezares B, Torres C, Castillo J, Saénz Y, et al. Resistencia a antibióticos y factores de virulencia en aislados clínicos de Salmonella entérica. Enferm Infecc Microbiol Clin. 2014;32:4-10.
- González-Abad MJ, Alonso-Sanz M. Incidencia y sensibilidad de Campylobacter jejunien pacientes pediátricos: implicación en bacteriemia. Rev Esp Quimioter. 2013;26:92-6.





