Vol. 15 - Num. 59
Consensus document
Consensus document on the aetiology, diagnosis and treatment of sinusitis
L Martínez Camposa, R Albañil Ballesterosb, J de la Flor Bruc, Roi Piñeiro Pérezd, J Cerverae, Fernando Baquero Artigaof, Santiago Alfayate Miguélezg, F Moraga Llopf, MJ Cilleruelo Ortegaa, Cristina Calvo Reyf
aSociedad Española de Infectología Pediátrica (SEIP).
bAsociación Española de Pediatría de Atención Primaria (AEPap).
ccSociedad Española de Pediatría Extrahospitalaria y de Atención Primaria (SEPEAP).
dServicio de Pediatría. Hospital Universitario General de Villalba. Collado Villalba. Madrid. España.
eSociedad Española de Otorrinolaringología Pediátrica (SEOP).
fSociedad Española de Infectología Pediátrica (SEIP). España.
gSección de Infectología Pediátrica. Hospital Clínico Universitario Virgen de la Arrixaca. Murcia. España.
Reference of this article: Martínez Campos L, Albañil Ballesteros R, de la Flor Bru J, Piñeiro Pérez R, Cervera J, Baquero Artigao F, et al. Consensus document on the aetiology, diagnosis and treatment of sinusitis. Rev Pediatr Aten Primaria. 2013;15:203-18.
Published in Internet: 29-08-2013 - Visits: 272583
Abstract
The Spanish National Consensus (Spanish Society of Pediatric Infectious Diseases, Spanish Association of Primary Care Pediatrics, Spanish Society of Pediatric Outpatient and Primary Care, Spanish Society of Otorhinolaryngology and Cervical-Facial Pathology) on Sinusitisis presented. Rhinosinusitis is a difficult to diagnose and often unrecognised disease. The document discusses the aetiology, the clinical signs and symptoms, and the diagnostic criteria. A proposal for treatment is made based on the epidemiological situation in our country. Oral amoxicillin is the treatment of choice (80 mg/kg/day divided every 8 hours). Alternative treatment is proposed in special cases and when amoxicillin is not sufficient. The main complications are reviewed.
Keywords
● Amoxicilin ● Diagnosis ● Rhinosinusitis ● Sinusitis ● TreatmentNote:
INTRODUCTION
Sinusitis is defined as the inflammation of one or more paranasal sinuses that usually occurs as a complication of a viral upper respiratory tract infection. When the symptoms last more than ten days, the presence of a bacterial superinfection is assumed. It is generally diagnosed based on clinical criteria, and although it is usually a self-limiting disease, it is the third leading cause of antibiotic prescription in Primary Care (following otitis and tonsillitis), despite being an underdiagnosed process that is often undocumented.
Areas of debate on sinusitis include its definition and identification, the involvement of viral or bacterial infections and non-infectious factors in its clinical course, its diagnosis based on clinical criteria versus the usefulness of supplemental tests, and its management with antibiotics and other coadjuvant measures1.
Consistent with the methodology of other consensus documents, we have included the strength of the recommendation (A: strong evidence, B: moderate evidence, C: weak evidence) and the quality of the scientific evidence (I: randomised controlled trials, II: well-designed studies that are not randomised, III: expert opinions based on clinical experience or descriptive studies) of the proposed measures, following the grading system of the Infectious Disease Society of America.
DEFINITIONS
The American Academy of Pediatrics defined these processes in 2001 as2:
- Acute bacterial sinusitis: bacterial infection of the paranasal sinuses lasting less than 30 days whose symptoms resolve completely.
- Subacute sinusitis: bacterial infection of the paranasal sinuses lasting between 30 and 90 days. It presents a microbiology similar to that of acute sinusitis.
- Recurrent acute sinusitis: episodes of bacterial infection lasting less than 30 days each and separated by intervals of at least 10 asymptomatic days. The patient must present 3 episodes of acute sinusitis in 6 months, or 4 in 12 months.
- Chronic sinusitis: episodes of inflammation lasting more than 90 days. Patients have persistent residual respiratory symptoms such as cough, rhinorrea, or nasal obstruction.
- Acute bacterial sinusitis superimposed on chronic sinusitis: patients develop new symptoms that resolve with antimicrobial treatment, but the underlying residual symptoms persist.
The latest international clinical practice guidelines have adopted the term “rhinosinusitis” by consensus to refer to the acute, subacute, or chronic inflammation regardless of its aetiology, as the mucosal lining of the nose and sinuses is contiguous and the sinuses are not affected without antecedent or concomitant inflammation of the nasal mucosa. At any rate, at present the old term “sinusitis” is still used interchangeably with the new one to refer to either entity3.
EPIDEMIOLOGY
According to United States statistics3, acute rhinosinusitis affects approximately 31 million patients (adults and children) a year, impacting the quality of life and the use of healthcare resources, and is the cause of a high volume of drug prescriptions. It is estimated that every year 1% of all children are bound to have sinusitis, which will result in a significant cost both in healthcare and in antibiotic consumption. Since some sinuses are pneumatised at birth, sinusitis can develop in infants, yet paediatricians often do not take into consideration this diagnosis in children younger than one year4.
In Spain we do not have available actual statistics on the incidence of rhinosinusitis, but if we infer that the situation is similar to that of other developed countries, and keeping in mind that children will have about 3 to 8 respiratory infections a year, we can predict that its impact in healthcare and drug prescriptions will not be insignificant5.
PATHOPHYSIOLOGY
We must consider a series of aspects, such as: a) the anatomy and development of the paranasal sinuses in children, b) the role of the nasal mucosa in viral or bacterial infections, and c) predisposing or exacerbating factors.
The paranasal sinuses in children
The paranasal sinuses are divided in 5 groups according to their location and drainage sites: the anterior and posterior ethmoidal sinuses, which drain in the middle and superior meatus, respectively; the two maxillary sinuses, which drain in the medium meatus, and the frontal sinuses6:
- The ethmoidal sinuses can be seen at birth, develop rapidly up to 7 years of age, and complete their development by 15-16 years of age.
- The maxillary sinuses are pneumatised at birth, reaching a volume of 2 ml by 2 years of age, and of around 10 ml by 9 years. They stop developing by 15 years of age.
- The frontal sinuses are indistinguishable from the anterior ethmoidal cells and grow so slowly that they cannot be identified anatomically until a year after birth. After the fourth year of life, they become larger and at six years they can be identified radiographically in 20 to 30% of children. They continue to develop during adolescence and at 12 years of age pneumatisation of these sinuses can be seen in computer tomography (CT) scans in 85% of children.
- The sphenoidal sinus is a very small evagination of the sphenoethmoidal recess. By seven years of age it has extended posteriorly to the level of the sella turcica, and by 8 years of age it appears pneumatised in CT scans in 85% of patients. Its growth is complete by 15 years of age1.
The sinonasal mucosa
The sinonasal mucosa has specific functions, such as filtering and warming up aspirated air, and the immune response to allergies, environmental irritants, and other particles to protect the delicate structure of the lower respiratory tract. It has been demonstrated that the sinusal mucosa is involved in the viral infection of the upper respiratory tract, which resolves spontaneously in most cases (acute viral rhinosinusitis), although in some cases an obstruction of the ostiumoccurs where air in the cavity is drawn in by the negative pressure facilitating the aspiration of nasopharyingeal mucus rich in bacteria (acute postviral rhinosinusitis) that contaminates the paranasal sinuses (which are sterile under normal conditions). This in turn can lead to bacterial propagation if the mucociliary apparatus does clear the mucus, and to the development of a bacterial infection of the sinuses (acute bacterial rhinosinusitis), which happens in 6-10% of cases1,7,8.
Predisposing or exacerbating factors
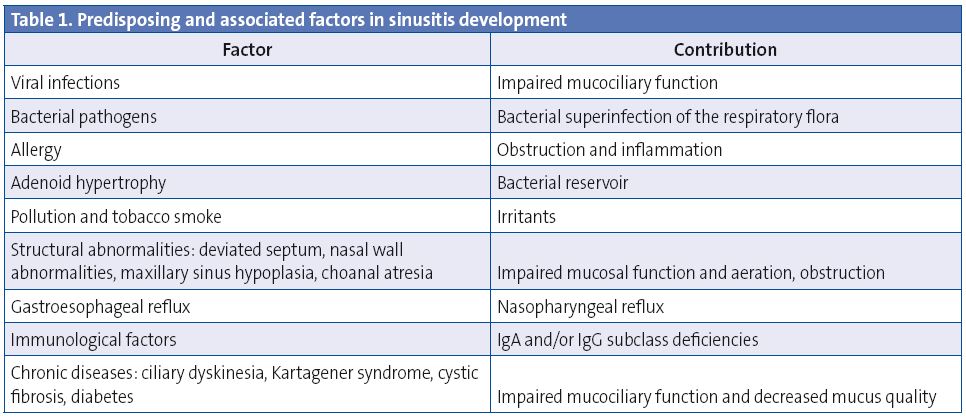
The inflammation of the sinonasal mucosa results from the interaction between the insulting agent (infectious or non-infectious), the local defence factors, and the host system, and there are some predisposing factors1,9,10 (Table 1).
AETIOLOGY
The factors that influence the development of bacterial rhinosinusitis are, among others, the nasopharyngeal microbiota, immunisation status, and previous treatment with antibiotics.
Normal nasopharyngeal flora
The nose is colonised by a polymicrobial flora, which studies with children have shown to include the species Streptococcus pneumoniae (S. pneumoniae) (50-60%), nontypeable Haemophilus influenzae (H. influenzae) (40-68%), Moraxella catarrhalis (M. catarrhalis) (34-50%) and, to a lesser degree, Streptococcus viridans, Streptococcus pyogenes (S. pyogenes) and Neisseria spp.11. The percentages are higher in children who have undergone a tonsillectomy12. The presence of this microbiota in asymptomatic children reinforces the low reliability of meatal cultures for the purpose of aetiological diagnosis.
Bacteria involved in acute rhinosinusitis
Most sinus infections are viral, and only a small proportion of cases develop a secondary bacterial infection5. Rhinovirus, influenza, and parainfluenza are the most common causes of acute rhinosinusitis. The paranasal sinuses are sterile under physiological conditions9, so the culture of paranasal sinus puncture samples would be the most suitable test for aetiological diagnosis. In the few studies that evaluated the application of this technique on children, S. pneumoniae was isolated in 35-42% of cultures, H. influenzae, in 21-28%, M. catarrhalis, in 21-28%, S. pyogenes, in 3-7%, and anaerobic microorganisms (in chronic and odontogenic processes), in 3-7%13-16. There is also the possibility of bacterial coinfection and that different bacterial distributions are involved in infections that involve multiple sinuses17.
Impact of immunisation against pneumococcus
Some studies have assessed the impact of the alterations in the microbiome brought upon by the introduction of pneumococcal conjugate vaccines in the aetiology of respiratory infections:
- Children show a decrease in nasal and oropharyngeal colonisation by S. pneumoniae with a relative increase in the presence of nontypeable H. influenzae18.
- The herd immunity resulting from immunisation is manifested in the aetiology of sinusitis in adults, with a 10% decrease in the recovery of S. pneumoniae, and a change in its identified serotypes, along with a 6% increase in H. influenzae19.
Studies assessing the impact of the introduction of the 13-valent conjugated pneumococcal vaccine on rhinosinusitis need to be done, as the published studies refer to the heptavalent vaccine.
Antibiotic resistance
The prevalence of penicillin resistance in S. pneumoniae ranges from 10 to 30%, and the prevalence of macrolide resistance is around 25%, with geographical variations and changes caused by the introduction of routine immunisations, as there has been a decrease in penicillin resistance following the introduction of the 13-valent conjugated pneumococcal vaccine (this vaccine is not included in the unified immunisation schedule presented by the Ministry of Health, but it is included in the recommendations of, CAV-AEP (Advisory Committee on Vaccines of the Spanish Association of Paediatrics)20-25. Macrolide resistance has been decreasing, from 26.4 to 20%, while resistance to levofloxacin has increased from 0.1 to 1.3% (2007), both in relation to the use of these antibiotics (greater and lesser, respectively). The production of beta-lactamases by H. influenzae has decreased in a sustained manner from 33 to 17.4% in recent years26 with the occasional appearance of ampicillin resistance in some strains that is not beta-lactamase-dependent 27, while 90 to 100% of M. catarrhalis strains still produce beta-lactamases28.
CLINICAL PRESENTATION
The most frequent symptoms of bacterial rhinosinusitis are nasal congestion, usually bilateral, nasal discharge of any type, consistency, and colour, and persistent cough, which may get worse at night. There may be vomiting caused by postnasal drip29. Other symptoms may include facial pain or pressure, which may be felt in the teeth, upper jaw, eyes, forehead, or one side of the face, and get worse when bending forward (pain is generally less prevalent in children)30. There may also be hyposmia or anosmia, and periocular swelling. Younger children may show less specific symptoms, such as irritability or poor appetite31. Preschool children may present with halitosis, otalgia and odynophagia, as well as wheezing. A headache could be the only symptom in some patients (sphenoiditis), but headache and facial pain in isolation of other symptoms are not specific signs of sinusitis.
Symptoms that suggest the development of complications include periorbital oedema, ocular motility abnormalities, recurring fever, severe headache, vomiting, mental state alterations, convulsions, focal neurological signs, and signs of increased intracranial pressure29,32.
DIAGNOSIS
The gold standard diagnostic for bacterial rhinosinusitis is the isolation of ≥104 colony-forming units from the culture of a sinus puncture aspirate33, but this procedure is not performed routinely—nor should it be—in clinical practise.
Bacterial sinusitis must be diagnosed based on clinical criteria, and supplemental testing must be reserved in case of suspected complications, poor response to treatment, recurrence of the disease, or special clinical situations such as immunodeficiency or severe underlying disease.
Three types of presentation are defined for the diagnosis of sinusitis34:
- Persistent catarrhal symptoms: congestion or nasal discharge, cough, or both, lasting for more than 10 days and less than 30 with no improvement (IIB). Nasal discharge could be watery, unusually mucoid, or purulent, while the cough could be either dry or productive and get worse at night. This is the most frequent presentation of acute bacterial sinusitis.
- Sudden onset of severe symptoms, essentially a high fever (≥39 °C) lasting more than 3-4 days, and purulent nasal discharge (IIB).
- Worsening of symptoms in the course of a common cold, with an increase in nasal discharge, daytime cough, or the development or recurrence of fever, especially if symptoms worsen starting at 6-7 days from the initial onset (IIB).
In 70% of school-children who develop a cold, some symptoms persist after 10 days, but they have improved35. The latest guidelines (American and European) agree that the persistence, severity, and worsening of symptoms are the key diagnostic criteria for the disease. However, they warn of the impossibility of differentiating viral from bacterial sinusitis accurately based solely on clinical signs and symptoms, which makes it difficult to select patients who could be given antibiotic therapy and assessed for its results, a difficulty that stems mostly from the lack of well-defined inclusion criteria for patient selection in research studies1,34.
PHYSICAL EXAMINATION
It does not usually help make the diagnosis, as possible findings may be absent, have low specificity, and do not differentiate between a viral and a bacterial aetiology 36. The nasal mucosa may have an erythemathous or pale appearance, there may be nasal discharge in the nasal fossae, mucus in the posterior wall of the pharynx, and pharyngeal and tympanic membrane erythema. There may be a soft and painless periorbital swelling. Palpation of the frontal and maxillary sinuses may cause pain, but facial pain is a low-sensitivity and low-specificity finding. The presence of halitosis in the absence of pharyngitis, of a foreign body, or poor dental hygiene may lead to suspecting sinusitis.
SUPPLEMENTAL TESTING
Routine supplemental testing is not recommended in case of presumed acute rhinosinusitis without complications.
Imaging tests
- Plain radiography of the sinuses has been used traditionally as a diagnostic test, but this is a sensitive but low-specificity test in the paediatric population. The signs found most frequently, sinus opacification and mucosal hypertrophy greater than 4 mm, are of little predictive value for a positive diagnosis, as they are found regularly in children who are healthy or have a common cold, viral rhinosinusitis, or allergic rhinitis37. Also, 35-50% of healthy children one to nine years of age and up to 97% of patients with a current or recent cold get false positives38. Hydroaerial levels, which have a higher specificity, are an infrequent finding37,38. Radiography should only be considered in cases of therapeutic failure or severe symptoms with suspected intracranial complications.
- CT is more reliable, but it can also give abnormal results in children with mild cold symptoms without clinical evidence for sinusitis, it often requires sedation, and it involves a greater exposure to radiation than plain radiography. Still, if an imaging test is required, this technique offers the best diagnostic performance39. A CT scan must be done on an urgent basis in case of proptosis, ocular motility or vision abnormalities, severe headache, repeated vomiting, convulsions, or sensory abnormalities.
- Magnetic resonance imaging (MRI) is very costly, and often requires sedation. It is worse than CT at demonstrating the bony structure of the osteomeatal complex, and it also shows abnormalities in patients with a common cold40, although it is more sensitive in the early detection of intracranial complications, in the differentiation between inflammation and tumours, and in cases of chronic fungal sinusitis, which is very rare in children41.
We may conclude that routine imaging is not recommended for evaluating acute bacterial sinusitis without complications in the paediatric population, and that it should be reserved for evaluating persistent, recurrent, or chronic sinusitis, or in case of suspected complications.
Other supplemental tests
- Endoscopic sinus examination has been shown to correlate adequately with CT findings, but cannot be performed on a routine basis42.
- Transillumination or diaphanoscopy is of little value in the paediatric population, as the sinuses are small and the findings are difficult to evaluate and apply only to the maxillary sinuses43.
- Portable ultrasound imaging of the paranasal sinuses is much more promising, but it is still relatively unknown and not widely practised. It is a quick, simple and non-invasive test. The procedure is painless, can be repeated as many times as needed, and does not require exposure to radiation. When performed expertly, this technique has shown much higher sensitivity (>86%) and specificity (>96%) than radiography in detecting the presence of secretions in the maxillary sinuses. However, it also has its limitations: it cannot be used for diagnosing ethmoidal or sphenoidal sinusitis, and the elevated cost of the machine poses a challenge to its systematic addition to the paediatrician’s office44-46.
DIFFERENTIAL DIAGNOSIS
The following conditions are considered in differential diagnosis:
- Common cold and acute rhinitis: usually, there is no fever or a short-lived low-grade fever, and the cough and nasal discharge start improving by the fifth or sixth day from onset. In sinusitis, these symptoms do not improve, there may be general malaise, and the fever (should it be present) and other symptoms are more severe and persistent. It may be difficult to differentiate sinusitis from recurrent colds, which are quite frequent in children, although asymptomatic intervals must occur in case of recurrent colds1,29.
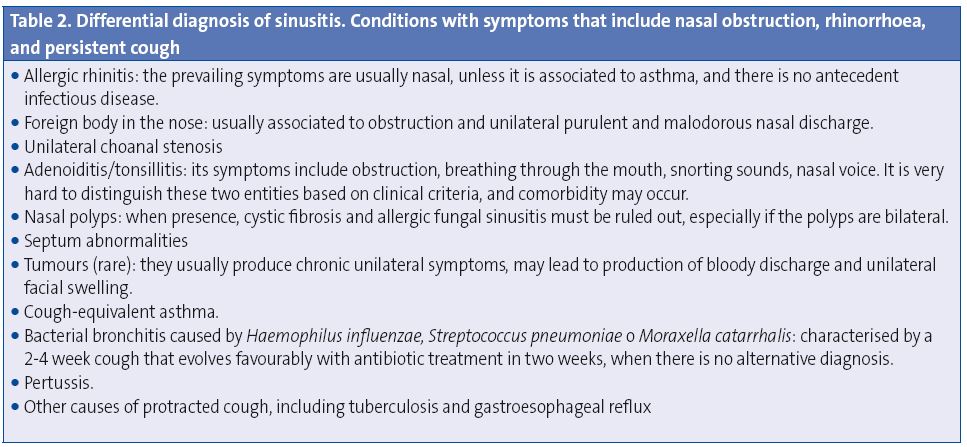
- Conditions with symptoms that include nasal obstruction, nasal discharge, and persistent cough1,47,48. These are detailed in Table 2.
- Conditions with symptoms that include with facial or cranial pain, such as tension headache, toothache, atypical facial pain, and temporomandibular joint dysfunction49. Predisposing factors and underlying primary conditions must be ruled out in case of recurrent or atypical clinical courses (Table 1).
REFERRAL CRITERIA
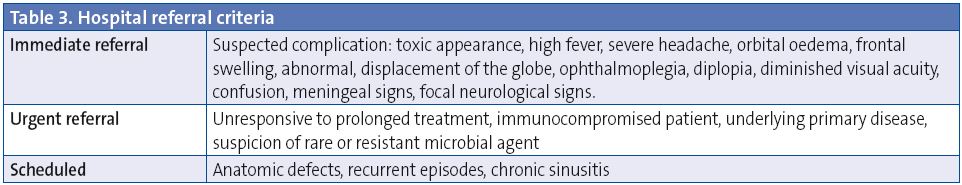
Referral criteria are noted in Table 3.
COMPLICATIONS OF SINUSITIS
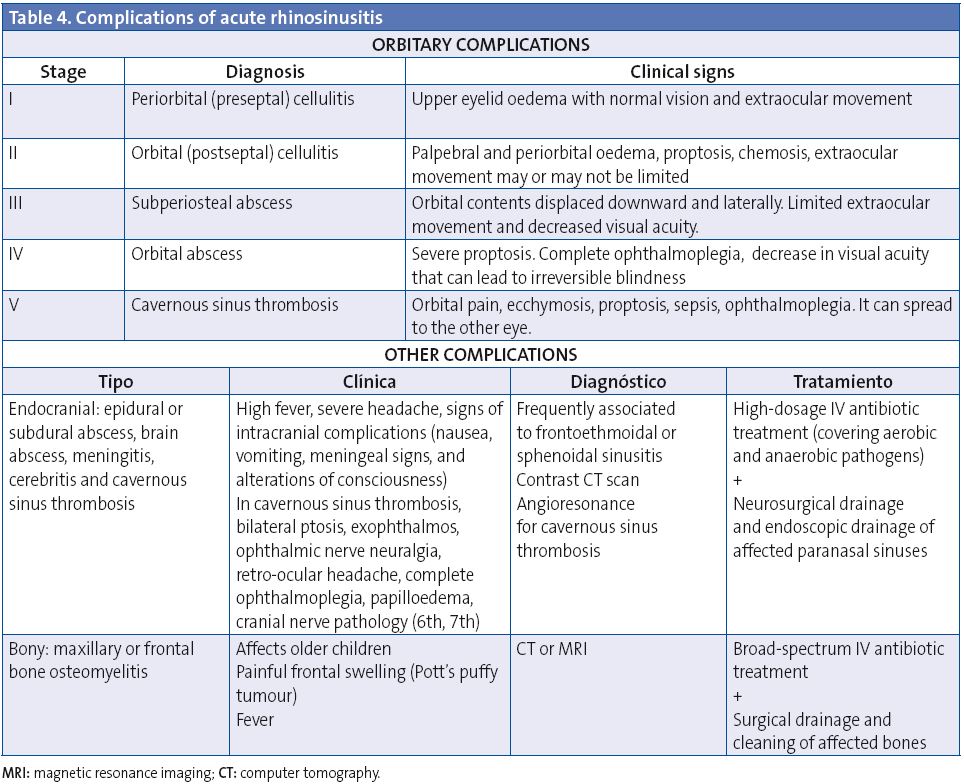
Complications develop in 3.7-11% of acute bacterial sinusitis cases and can be classified as orbital (60-70%), intracranial (15-20%), and bony (5-10%) (Table 4). Orbital complications develop most often between 3 and 6 years of age, and intracranial complications are more frequent in adolescents50,51. The most common complication of acute rhinosinusitis is periorbital cellulitis.
Orbital complications
Infection may spread easily to the orbit directly through the lamina papyracea, which is very thin and may be dehiscent; it can also spread along venous channels52. Chandler et al.53 did the classification of orbital complications in 1970, a system that organises the typical models of orbital pathology with their expected disease progression (Table 4). It is important to be aware that orbital complications may be painless in children.
Periorbital or preseptal cellulitis
It is the swelling of the eyelid and the conjunctiva, which affects the tissue anterior to the orbital septum and can be discerned easily in a CT as a soft-tissue inflammation. It often occurs as a complication of upper respiratory tract infection, dacryocystitis or skin infection, and sinusitis54. It presents with palpebral oedema, erythema, and fever. It is not associated to proptosis, and it does not lead to limited ocular motility. It usually responds well to antibiotic therapy, but if it is not treated early on it can spread beyond the orbital septum. In most cases, preseptal cellulitis is a clinical diagnosis that does not require evaluation with a CT scan.
Orbital or postseptal cellulitis
It develops as inflammatory changes affect the orbit, and symptoms include chemosis, proptosis, and limited and painful eye movements56. This complication requires intensive treatment with intravenous antibiotic therapy and ruling out the presence of a subperiosteal or orbital abscess by means of CT. If intracranial complications are suspected, the evaluation must also include MRI.
Subperiosteal and orbital abscess
Subperiosteal abscesses form between the periorbita and the paranasal sinuses in the outermost part of the extraocular muscles. The clinical signs include swelling, erythema, ecchymosis, and proptosis, with decreased ocular motility (ophthalmoplegia) and diminished visual acuity57.
Orbital abscesses are intraconal, limited by the recti muscles and their sheaths, and by Tenon’s capsule. They usually develop in cases of delayed diagnosis or in immunocompromised patients, and their prevalence ranges between 8 and 13%58.
Surgical drainage is indicated when there is an abscess confirmed by CT and progressive vision loss or absence of symptom improvement after 48 hours of intravenous therapy59. An ophthalmologist must monitor visual acuity, and the patient can be switched to oral antibiotic therapy once fever has been absent for over 48 hours and ophthalmological signs and symptoms have resolved.
The current consensus guidelines recommend that preseptal and orbital cellulitis be treated with antibiotic therapy first, with surgical intervention required for subperiosteal and intraorbital abscesses, usually by endoscopy57. However, there are recent studies that show good results with intravenous antibiotics in children with subperiosteal abscesses60 when the following conditions are met: improvement of symptoms in 24-48 hours, normal visual acuity; small subperiosteal abscess (<0.5 to 1 ml) located medially, absence of systemic disease, and patient age between 2 and 4 years61.
MANAGEMENT
Non-antibiotic treatment
The use of vitamin C, zinc, echinacea, decongestants, systemic antihistamines, or mucolytics is not recommended in the latest reviews due to a lack of efficacy and/or potential toxicity62,63.
Isotonic or hypertonic saline solutions result in a self-reported improvement of the symptoms and of mucociliary clearance64-66, improve the elimination of secretions, and prevent crust formation, but the data are still too few to make a recommendation based on strong-enough evidence67. Oral corticosteroids as adjuvant treatment with antibiotic treatment are effective in the short-term alleviation of acute sinusitis symptoms. Still, the data are limited and there are no quality studies justifying their use as a monotherapy or as an adjuvant treatment in antibiotic therapy68.
Intranasal corticosteroids seem to be somewhat useful in combination with antibiotics, especially in studies done with adults, and could be beneficial in children with underlying allergic rhinitis, but there need to be more studies supporting their usefulness in sinusitis in the paediatric population69.
Antibiotic treatment
In 2001, the AAP recommended the use of antibiotics in acute bacterial sinusitis, although its efficacy in symptom control and above all in preventing the development of potential complications of the disease remains the subject of heated controversy1,34,70-75. There is a high rate of spontaneous resolution of uncomplicated acute sinusitis (60-80%), so at present the trend is to recommend antibiotic prescription only for persistent or complicated cases. The consensus group also recommends starting antibiotic therapy whenever the diagnostic criteria for bacterial sinusitis are met (see the section on diagnosis), except in children whose symptoms have lasted at least 10 days but are showing improvement. In these cases, the approach would be one of watchful waiting, monitoring the patient closely and treating the symptoms.
First-line treatment
Our first-line treatment is amoxicillin76-78, which shows good activity against pneumococcus, the pathogen most frequently involved and causing the highest rate of complications. In areas with high rates of immunisation against pneumococcus, we have observed a decrease in nasopharyngeal colonisation by pneumococcus and an increase in nontypeable H. influenzae and M. catarrhalis isolates. In such instances, amoxicillin-clavulanic acid can be used as an alternative, as most M. catarrhalis isolates and 10-20% of H. influenzae isolates produce beta-lactamases. Amoxicillin-clavulanic acid is also recommended in sinusitis with a high risk for complications where all possible conditions need to be covered: children younger than 2 years, frontal or sphenoidal sinusitis, complicated ethmoidal sinusitis, patients with very severe or protracted symptoms (lasting longer than a month), patients with chronic conditions or who are immunocompromised, or patients who did not respond to initial treatment with amoxicillin.
In Spain, the recommended dose of amoxicillin (be it alone or in combination with clavulanic acid) is 80-90 mg/kg/day divided at 8-hour intervals, as the resistance to penicillin rates of pneumococcus are greater than10%.
Alternative treatment
Various studies have shown that second-generation oral cephalosporins (cefuroxime axetil), and third-generation cephalosporins (cefpodoxime proxetil o ceftibuten) and fluoroquinolones are efficacious, but the results were not better than those achieved with amoxicillin or amoxicillin-clavulanic acid79,80, so their use should be reserved for patients with a non-severe (not Type I) hypersensitivity to penicillin. Although macrolides are not a good treatment option due to the high percentage of resistance to these drugs (25-30%), in case of immediate or rapid (Type I) hypersensitivity and low-severity disease they can be used while reserving other treatment options. Another option when these patients have mild symptoms is to monitor them carefully and delay initiation of antibiotic therapy. Levofloxacin can be used as a last option for this particular group of children with severe Type I allergy to penicillin and poor response to treatment with macrolides.
Duration of antibiotic therapy
It is recommended that antibiotic treatment lasts between 7 and 14 days1,32, with 10 days being the duration recommended most frequently76,77,81,82. Some patients respond slowly to treatment and require longer courses, in which case it is recommended to extend antibiotic therapy until 7 days after symptoms resolve. In some cases (children with partial responses to treatment), the therapy can extend to up to 3 weeks76,81.
Recommended approach in case of therapeutic failure
With adequate treatment, in 48-72 hours children no longer have a fever and the coughing and rhinorrhoea are gradually subsiding76,82. If this is not the case, the diagnosis and treatment must be re-evaluated76,82. The main causes of treatment failure, once it is ascertained that the treatment was administered correctly, are: microbial resistance to the antibiotic used, development of complications, non-infectious aetiology (intranasal foreign bodies, structural malformations, and allergy) or, in rare cases, the existence of a chronic condition or an immunodeficiency. If microbial drug resistance is suspected, it is advisable that the empiric antibiotic therapy is modified by adding an antimicrobial that is efficient against beta-lactamase-producing bacteria or pneumococci with high-level penicillin resistance: amoxicillin-clavulanic acid, or even third-generation cephalosporins, respectively (intramuscular ceftriaxone)76,82.
Criteria for hospital admission and selection of empiric intravenous antibiotic therapy
Children showing signs of sepsis or deterioration of their general health status, whose treatment with oral drugs has failed, or who have developed complications (to be considered in case of preseptal cellulitis) must be hospitalised and treated parenterally with one of the following antibiotics: amoxicillin-clavulanic acid, cefotaxime or ceftriaxone76,82.Imaging is recommended for confirmatory diagnosis and for evaluation by an otorhinolaryngology specialist (ORL). If intracranial complications with the presence of anaerobic bacteria are suspected, cefotaxime must be administered in conjunction with metronidazole. In case of Type I penicillin allergy, levofloxacin in conjunction with metronidazole may be an option for patients with severe disease.
Treatment protocol
- Non-antibiotic medical treatment:
- Pain relief: recommended (IA). Ibuprofen or paracetamol administered orally at regular doses. Ibuprofen shows a better time course of action due to its double analgesic and anti-inflammatory properties.
- Saline solution washes: recommended therapy (IIB). It is an inexpensive and innocuous treatment that has proven useful in some studies.
- Intranasal corticosteroid therapy: recommended in children with underlying allergic rhinitis (IIIC); and in children with no underlying allergic condition (IIIC), especially in patients who are being monitored without antibiotic treatment.
- Mucolytics, decongestants, and antihistamines: not recommended (IA).
- Monitoring without antibiotic treatment: it is recommended to delay initiating treatment with antibiotics in children whose symptoms have lasted more than ten days but are showing improvement.
- Oral antibiotic treatment: recommended in all other patients (IIB):
- First-line treatment:
- Amoxicillin: 80-90 mg/kg/day in divided doses every 8 hours for 10 days (IIB).
- In children younger than two years with sphenoid or frontal sinusitis, incipient preseptal cellulitis, who are immunocompromised, or have a significant underlying condition, with very severe or protracted symptoms (lasting more than one month), and whenever the patient does not seem to respond to initial treatment with amoxicillin:
- Amoxicillin-clavulanic acid (8/1): 80-90 mg/kg/day in divided doses every 8 hours for 10 days (IIB).
- In children with delayed hypersensitivity (non-anaphylactic reaction):
- Cefpodoxime proxetil: 10 mg/kg/day doses in divided doses every 12 hours for 10 days (IIB).
- Ceftibuten: 9 mg/kg/day doses every 24 hours (maximum of 400 mg/day), for 5-10 days (IIIC).
- Cefuroxime axetil: 30 mg/kg/day in divided doses every 12 hours for 10 day (IIB).
- In children with immediate or rapid hypersensitivity to penicillin (Type I, anaphylactic reaction):
- Strongly consider the option of watchful waiting without antibiotic treatment (IIIC).
- In non-severe cases, clarithromycin 15 mg/kg/day divided in doses every 12 hours (IIIC) or azithromycin 10 mg/kg/day every 24 hours for 3 days, or 10 mg/kg/day the first day and 5 mg/kg/day for four more days.
- In severe cases or if treatment with macrolides has failed, levofloxacin, 10-20 mg/kg/day in divided doses every 12-24 hours for 10 days (off-labeluse) (IIIC).
- In children with poor tolerance to the initial oral treatment:
- Intramuscular ceftriaxone intramuscular in 50 mg/kg/day doses every 24 hours for 1-3 days, followed by one of the treatment courses noted above (depending on clinical history) until completion of 10 days of treatment (IIIC). Ceftriaxone is a hospital-only drug, so the patient must be referred to the hospital to evaluate its administration.
- First-line treatment:
- Recommended approach in case of therapeutic failure after 48-72 hours of correct initial antibiotic treatment:
- Differential diagnosis:
- Complications.
- Non-infectious aetiology.
- Immunodeficiencies.
- Consider imaging tests in case of complications (IIIC).
- Change of empiric oral antibiotic determined by the antibiotic used initially (IIB):
- Amoxicillin-clavulanic acid: 80-90 mg/kg/day divided in doses every 8 hours if treatment started with amoxicillin (IIB).
- Oral cephalosporins (cefuroxime o ceftibuten) provide benefits beyond those of treatment with amoxicillin-clavulanic acid, so they are not recommended in this consensus in case of initial treatment failure.
- Levofloxacin in 10 mg/kg doses every 12 hours in children aged 6 months to 5 years, and in 10 mg/kg doses every 24 hours in children older than five years (maximum dose 500 mg/day) (off-labeluse) in children with Type I penicillin allergies (anaphylaxis) if treatment with macrolides has not been effective (IIIC).
- Patients who have not improved with the treatments indicated above should be referred to the hospital to receive intramuscular ceftriaxone.
- Differential diagnosis:
- Criteria for hospital referral and treatment:
- Criteria for hospital admission:
- Signs of sepsis.
- Deterioration of general health status.
- Persistent failure after two rounds of oral treatment (criterion for evaluation at the hospital with or without admission).
- Complications (with the possible exception of preseptal cellulitis).
- High-risk household environment where compliance with treatment is not guaranteed.
- Recommended radiographic tests (IIB).
- Assessment by ORL and Ophthalmology specialists in case of orbital or periorbital cellulitis (IIIC).
- Intravenous treatment (IIB):
- Amoxicillin-clavulanic acid: 100 mg/kg/day in divided doses every 6 hours (IIB).
- Cefotaxime: 150-200 mg/kg/day in divided doses every 6-8 hours (IIB) or ceftriaxone, 50-100 mg/kg/day in divided doses every 12-24 hours (IIIC), if the patient had been treated previously with amoxicillin-clavulanic acid.
- Levofloxacin in 10 mg/kg doses every 12 hours in children 6 months to 5 years of age, and 10 mg/kg doses every 24 hours in children older than 5 years (maximum dose 500 mg/day) (off-label use) (IIIC), in children with a Type I penicillin allergy.
- In case of suspected intracranial complications or possible infection with anaerobes:
- Add metronidazole to treatment with cefotaxime (or levofloxacin in allergic patients), 30 mg/kg/day in divided doses every 6 hours (IIIC).
- If intravenous antibiotic treatment fails, potential complications must be evaluated in consultation with the ORL specialist and an expert in paediatric infectology.
- Criteria for hospital admission:
CONFLICT OF INTERESTS
Doctors Martínez, Baquero, Calvo, de la Flor, Alfayate, Cilleruelo, and Moraga have collaborated as speakers in conferences or researchers in studies funded by one of the following organisations: Wyeth/Pfizer, Sanofi-Pasteur-MSD, GlaxoSmithKline, Novartis, Crucell, Esteve, Abbvie, and Astra-Zeneca. The rest of the authors declare having no conflicts of interests.
ACRONYMS: ORL: Otorhinolaryngology • MRI: nuclear magnetic resonance imaging • CT: computer tomography.
BIBLIOGRAPHY
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;50(Supl 23):1-298.
- American Academy of Pediatrics. Subcommittee on Management of Sinusitis and Committee on Quality Improvement. Clinical practice guideline: management of sinusitis. Pediatrics. 2001;108(3):798-808. Erratum in: Pediatrics. 2002;109(5):40. Pediatrics. 2001;108(5):A24.
- Meltzer EO, Hamilos DL. Rhinosinusitis diagnosis and management for the clinician: a synopsis of recent consensus guidelines. Mayo Clin Proc. 2011;86(5):427-43.
- McQuillan L, Crane LA, Kempe A. Diagnosis and management of acute sinusitis by pediatricians. Pediatrics. 2009;123(2):e193-8.
- Tomás M, Ortega P, Mensa J, García J, Barberán J. Diagnóstico y tratamiento de las rinosinusitis agudas. Segundo consenso. Rev Esp Quimioter. 2008;21(1):45-59.
- Jones N. The nose and paranasal sinuses physiology and anatomy. Adv Drug Deliv Rev. 2001;51(1-3):5-19.
- Gwaltney JM. Clinical significance and pathogenesis of viral respiratory infections. Am J Med. 2002;112 Suppl 6A:13S-18S.
- Marchisio P, Ghisalberti E, Fusi M, Baggi E, Ragazzi M, Dusi E. Paranasal sinuses and middle ear nfections: what do they have in common? Pediatr Allergy Immunol. 2007;18 Suppl 18:31-4.
- Beule AG. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. Laryngorhinootologie. 2010;89 Suppl 1:S15-34.
- Goldsmith AJ, Rosenfeld RM. Treatment of pediatric sinusitis. Pediatr Clin North Am. 2003;50(2):413-26.
- Gordts F, Halewyck S, Pierard D, Kaufman L, Clement PA. Microbiology of the middle meatus: a comparison between normal adults and children. J Laryngol Otol. 2000;114(3):184-8.
- Gordts F, Abu Nasser I, Clement PA, Pierard D, Kaufman L. Bacteriology of the middle meatus in children. Int J Pediatr Otorhinolaryngol. 1999;48(2):163-7.
- Brook I. Management of Bacterial Rhinosinusitis in Children. Eur Respir Dis. 2012;8(1):56-60.
- Brook I. Microbiology of sinusitis. Proc Am Thorac Soc. 2011;8(1):90-100.
- Brook I. Bacteriology of acute and chronic ethmoid sinusitis. J Clin Microbiol. 2005;43(7):3479-80.
- Wald ER. Microbiology of acute and chronic sinusitis in children and adults. Am J Med Sci. 1998;316(1):13-20.
- Brook I. Discrepancies in the recovery of bacteria from multiple sinuses in acute and chronic sinusitis. J Med Microbiol. 2004;53(Pt 9):879-85.
- Brook I, Gober AE. Frequency of recovery of pathogens from the nasopharynx of children with acute maxillary sinusitis before and after the introduction of vaccination with the 7-valent pneumococcal vaccine. Int J Pediatr Otorhinolaryngol. 2007;71(4):575-9.
- Brook I, Foote PA, Hausfeld JN. Frequency of recovery of pathogens Rausing acute maxillary sinusitis in adults before and after introduction of vaccination of children with the 7-valent pneumococcal vaccine. J Med Microbiol. 2006;55(Pt 7):943-6.
- Moreno-Pérez D, Álvarez García FJ, Arístegui Fernández J, Barrio Corrales F, Cilleruelo Ortega MJ, Corretger Rauet JM, et al.; Comité Asesor de Vacunas de la Asociación Española de Pediatría, España. Calendario de vacunaciones de la AEP: recomendaciones 2013. An Pediatr (Barc). 2013;78(1):59.e1-27.
- Liñares J, Ardanuy C, Pallares R, Fenoll A. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-yearperiod. Clin Microbiol Infect. 2010;16(5):402-10.
- Fenoll A, Granizo JJ, Aguilar L, Giménez MJ, Aragoneses-Fenoll L, Hanquet G, et al. Temporal trends of invasive Streptococcus pneumoniaeserotypes and antimicrobial resistance patterns in Spain from 1979 to 2007. J Clin Microbiol. 2009;47(4):1012-20.
- Sánchez-Tatay D, Arroyo LA, Tarragó D, Lirola MJ, Porras A, Fenoll A, et al. Antibiotic susceptibility and molecular epidemiology of nasopharyngeal pneumococci from Spanish children. Clin Microbiol Infect. 2008;14(8):797-801.
- Fenoll A, Aguilar L, Vicioso MD, Giménez MJ, Robledo O, Granizo JJ. Increase in serotype 19A prevalence and amoxicillin non-susceptibility among paediatric Streptococcus pneumoniae isolates from middle ear fluid in a passive laboratory-based surveillance in Spain, 1997-2009. BMC Infect Dis. 2011;11:239.
- Picazo JJ. Management of antibiotic-resistant Streptococcus pneumoniae infections and the use of pneumococcal conjugate vaccines. Clin Microbiol Infect. 2009;15 Suppl 3:4-6.
- García-Cobos S, Campos J, Cercenado E, Román F, Lázaro E, Pérez-Vázquez M, et al. Antibiotic resistance in Haemophilus influenzae decreased, except for beta-lactamase-negative amoxicillin-resistant isolates, in parallelwith community antibiotic consumption in Spain from 1997 to 2007. Antimicrob Agents Chemother. 2008;52(8):2760-6.
- Aracil B, Gómez-Garcés JL, Alós JI; Grupo de Estudio de Infección en Atención Primaria de la SEIMC (IAP-SEIMC). Sensibilidad de Haemophilus influenzae aislados en España a 17 antimicrobianos de administraciónn oral. Enferm Infecc Microbiol Clin. 2003;21(3):131-6.
- Oteo J, Campos J. Valor de los sistemas de vigilancia de resistencia a antibióticos. Enferm Infecc Microbiol Clin. 2003;21(3):123-5.
- Wald E, Kaplan S, Friedman E, Wood R. Acute bacterial rhinosinusitis in children: Clinical features and diagnosis. UpToDate (update 12/6/2012) [on line] [consulted on 12/nov/2012]. Available on www.uptodate.com/contents/acute-bacterial-rhinosinusitis-in-children-clinical-features-and-diagnosis
- Pappas E, Hendley J. Sinusitis. In: Kliegman R, Behrman R, Jenson H, Stanton B. Nelson Tratado de Pediatría, 18.ª edición española. Barcelona: Elsevier; 2009. p. 1749-52.
- Mori F, Fiocchi A, Barni S, Beghi G, Caddeo A, Calcinai E, et al. Management of acute rhinosinusitis. Pediatr Allergy Immunol. 2012;23 Suppl s22:27-31.
- DeMuri GP, Wald ER. Complications of acute bacterial sinusitis in children. Pediatr Infect Dis J. 2011;30(8):701-2.
- Díez O, Batista N, Bordes A, Lecuona M, Lara M. Diagnóstico microbiológico de las infecciones del tracto respiratorio superior. Enferm Infecc Microbiol Clin. 2007;25(6):387-93.
- Chow AW, Benninger MS, Brook I, Brozek JL, Goldstein EJ, Hicks LA, et al. Infectious Diseases Society of America. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54(8):e72-e112.
- Pappas DE, Hendley JO, Hayden FG, Winther B. Symptom profile of common colds in school-aged children. Pediatr Infect Dis J. 2008;27(1):8-11.
- DeMuri, Wald E. Acute Sinusitis: Clinical Manifestations and Treatment Approaches. Pediat Ann. 2010;39:34-40.
- Manning SC, Biavati MJ, Phillips DL. Correlation of clinical sinusitis signs and symptoms to imaging findings in pediatric patients. Int J Pediatr Otorhinolaryngol. 1996;37(1):65-74.
- Glasier CM, Mallory GB Jr, Steele RW. Significance of opacification of the maxillary and ethmoid sinuses in infants. J Pediatr. 1989;114(1):45-50.
- American college of radiology ACR Aproppiateness criteria. 2012 [on line] [consulted on 26/abr/2013]. Available on www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/SinusitisChild.pdf
- von Kalle T, Fabig-Moritz C, Heumann H, Winkler P. Incidental findings in paranasal sinuses and mastoid cells: a cross-sectional magnetic resonance imaging (MRI) study in a pediatric radiology department. Rofo. 2012;184(7):629-34.
- McAlister WH. Imaging of sinusitis in infants and children. In: Lusk RP (ed.). Pediatric Sinusitis. New York, NY: Raven Press; 1992. p. 15-42.
- Castellanos J, Axelrod D. Flexible fiberoptic rhinoscopy in the diagnosis of sinusitis. J Allergy Clin Immunol. 1989;83(1):91-4.
- Otten FW, Grote JJ. The diagnostic value of transillumination for maxillary sinusitis in children. Int J Pediatr Otorhinolaryngol. 1989;18(1):9-11.
- Karantanas AH, Sandris V. Maxillary sinus inflammatory disease: ultrasound compared to computed tomography. Comput Med Imaging Graph. 1997;21(4):233-41.
- Tiedjen KU, Becker E, Heimann KD, Knorz S, Hildmann H. Value of B-image ultrasound in diagnosis of paranasal sinus diseases in comparison with computerized tomography. Laryngorhinootologie. 1998;77(10):541-6.
- de La Flor J, Parellada N. Correlació entre simptomatologia clínica sospitosa de sinusitis i presencia d’hipertròfia de mucosa i/o exsudat de sins maxillars, i d’exsudat de sins frontals, detectats amb ultrasonografia portátil en una consulta de Pediatría d’atenció primària. Pediatr Catalana. 2005;63:65-76.
- Acute Bacterial Sinusitis Guideline Team, Acute Bacterial Sinusitis Guideline Team, Cincinnatti Children’s Hospital MedicalCenter: Evidence-Based Care Guideline for medical management of Acute Bacterial Sinusitis in children 1 through 17 yearsof age [en línea]. Available on www.cincinnatichildrens.org/workarea/linkit.aspx?linkidentifier=id&itemid=87964&libid=87652
- Chang AB, Glomb WB. Guidelines for evaluating chronic cough in pediatrics: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(Suppl 1):260s-283s.
- Clinical Knowledge Summaries. NICE. Sinusitis. Additional information [on line] [consulted on 30/mar/2013]. Available on http://cks.nice.org.uk/sinusitis#!diagnosisadditional
- Kristo A, Uhari M. Timing of rhinosinusitis complications in children. Pediatr Infect Dis J. 2009;28(9):769-71.
- Sultész M, Csákányi Z, Majoros T, Farkas Z, Katona G. Acute bacterial rhinosinusitis and its complications in our pediatric otolaryngological department between 1997 and 2006. Int J Pediatr Otorhinolaryngol. 2009;73(11):1507-12.
- Rumelt S, Rubin PA. Potential sources for orbital cellulitis. Int Ophthalmol Clin. 1996;36(3):207-21.
- Chandler JR, Langenbrunner DJ, Stevens ER. The pathogenesis of orbital complications in acute sinusitis. Laryngoscope. 1970;80(9):1414-28.
- Georgakopoulos CD, Eliopoulou MI, Stasinos S, Exarchou A, Pharmakakis N, Varvarigou A. Periorbital and orbital cellulitis: a 10-year review of hospitalized children. Eur J Ophthalmol. 2010;20(6):1066-72.
- Sobol SE, Marchand J, Tewfik TL, Manoukian JJ, Schloss MD. Orbital complications of sinusitis in children. J Otolaryngol. 2002;31(3):131-6.
- Bayonne E, Kania R, Tran P, Huy B, Herman P. Intracranial complications of rhinosinusitis. A review, typical imaging data and algorithm of management. Rhinology. 2009;47(1):59-65.
- Coenraad S, Buwalda J. Surgical or medical management of subperiosteal orbital abscess in children: a critical appraisal of the literature. Rhinology. 2009;47(1):18-23.
- Jones NS, Walker JL, Bassi S, Jones T, Punt J. The intracranial complications of rhinosinusitis: can they be prevented? Laryngoscope. 2002;112(1):59-63.
- Gavriel H, Yeheskeli E, Aviram E, Yehoshua L, Eviatar E. Dimension of subperiosteal orbital abscess as an indication for surgical management in children. Otolaryngol Head Neck Surg. 2011;145(5):823-7.
- Siedek V, Kremer A, Betz CS, Tschiesner U, Berghaus A, Leunig A. Management of orbital complications due to rhinosinusitis. Eur Arch Otorhinolaryngol. 2010;267(12):1881-6.
- Hoxworth JM, Glastonbury CM. Orbital and intracranial complications of acute sinusitis. Neuroimaging Clin N Am. 2010;20(4):511-26.
- Gunn VL, Taha SH, Liebelt EL, Serwint JR. Toxicity of over-the-counter cough and cold medications. Pediatrics. 2001;108(3):E52.
- Shaikh N, Wald ER, Pi M. Decongestants, antihistamines and nasal irrigation for acute sinusitis in children. Cochrane Database Syst Rev. 2012 Sep 12;9:CD007909.
- Hauptman G, Ryan MW. The effect of saline solutions on nasal patency and mucociliary clearance in rhinosinusitis patients. Otolaryngol Head Neck Surg. 2007;137(5):815-21.
- Wang YH, Yang CP, Ku MS, Sun HL, Lue KH. Efficacy of nasal irrigation in the treatment of acute sinusitis in children. Int J Pediatr Otorhinolaryngol. 2009;73(12):1696-701.
- Wang YH, Ku MS, Sun HL, Lue KH. Efficacy of nasal irrigation in the treatment of acute sinusitis in atopic children. J Microbiol Immunol Infect. 2012 Sep 30. pii: S1684-1182(12)00179-X.
- Kassel JC, King D, Spurling GK. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database Syst Rev. 2010 Mar17;(3):CD00682.
- Venekamp RP, Thompson MJ, Hayward G, Heneghan CJ, Del Mar CB, Perera R, et al. Systemic corticosteroids for acute sinusitis. Cochrane Database Syst Rev. 2011 Dec 7;(12):CD008115.
- Zalmanovici A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2011 May 8(4):CD005149.
- Sinclair CF, Berkowitz RG. Prior antibiotic therapy for acute sinusitis in children and the development of subperiosteal orbital abscess. Int J Pediatr Otorhinolaryngol. 2007;71(7):1003-6.
- Falagas ME, Giannopoulou KP, Vardakas KZ, Dimopoulos G, Karageorgopoulos DE. Comparison of antibiotics with placebo for treatment of acute sinusitis: a meta-analysis of randomised controlled trials. Lancet Infect Dis. 2008;8(9):543-52.
- Blin P, Blazejewski S, Lignot S, Lassalle R, Bernard MA, Jayles D, et al. Effectiveness of antibiotics for acute sinusitis in real-life medical practice. Br J Clin Pharmacol. 2010;70(3):418-28.
- Guarch Ibáñez B, Buñuel Álvarez JC, López Bermejo A, Mayol Canalsa L. El papel de la antibioterapia en la sinusitis aguda: revisión sistemática y metaanálisis. An Pediatr (Barc). 2011;74:154-60.
- Wald ER. Treatment of acute sinusitis. Pediatr Infect Dis J. 2010;29(1):94.
- Hansen FS, Hoffmans R, Georgalas C, Fokkens WJ. Complications of acute rhinosinusitis in The Netherlands. Fam Pract. 2012;29(2):147-53.
- Méndez Hernández M, Rodrigo Gonzalo de Liria C. Sinusitis aguda. Celulitis periorbitaria. Protocolos de Infectología de la Asociación Española de Pediatría y la Sociedad Española de Infectología Pediátrica. Año 2011 [on line] [consulted on 15/oct/2012]. Available on www.aeped.es/sites/default/files/documentos/sinusitis.pdf
- American Academy of Pediatrics. Principles of appropriate use for upper respiratory tract infections. In: Pickering LK (ed.). Red Book. 2012. Report of the Committee on Infectious Diseases, 29th, Elk Grove Village, IL: American Academy of Pediatrics; 2012. p.802.
- Del Castillo Martín F, Baquero Artigao F, de la Calle Cabrera T, López Robles MV, Ruiz Canela J, Alfayate Miguelez S, et al. Documento de consenso sobre etiología, diagnóstico y tratamiento de la otitis media aguda. Rev Pediatr Aten Primaria. 2012;14:195-205 [on line] [consulted on 16/ago/2013]. Available on www.pap.es/files/1116-1515-pdf/iye_pap_55_02.pdf
- Kristo A, Uhari M, Luotonen J, Ilkko E, Koivunen P, Alho OP. Cefuroxime axetil versus placebo for children with acute respiratory infection and imaging evidence of sinusitis: a randomized, controlled trial. Acta Paediatr. 2005;94(9):1208-13.
- Karageorgopoulos DE, Giannopoulou KP, Grammatikos AP, Dimopoulos G, Falagas ME. Fluoroquinolones compared with beta-lactam antibiotics for the treatment of acute bacterial sinusitis: a meta-analysis of randomized controlled trials. CMAJ. 2008;178(7):845-54.
- Institute for Clinical Systems Improvement (ICSI). Diagnosis and treatment of respiratory illness in children and adults. Third Edition. January 2011[on line] [consulted on 15/oct/2012]. Available on www.icsi.org
- Wald ER, Kaplan SL, Isaacson GC, Wood RA, Torchia MM. Acute bacterial rhinosinusitis in children: Microbiology and treatment. UpToDate (updated 18/09/12) [on line] [consulted on 18/oct/2012]. Available on www.uptodate.com/home/





