Vol. 26 - Num. 104
Original Papers
Aetiology and antibiotic resistance in paediatric urinary tract infection. A multicentre study in primary care
M.ª Rosa Albañil Ballesterosa, Ana Cubero Santosb, M.ª José Martínez Chamorroc, M.ª Eulalia Muñoz Hiraldod, Josefa Ares Álvareze, Beatriz Morillo Gutiérrezf, M.ª Ángeles Suárez Rodríguezg, Rafael Jiménez Alésh
aPediatra. CS Cuzco . Fuenlabrada. Madrid. España. Miembro GPI AEPap.
bPediatra. CS San Roque. Badajoz. España. Miembro GPI AEPap.
cPediatra. CS Polanco. Cantabria. España. Miembro GPI AEPap.
dPediatra. CS Dr. Castroviejo. Madrid. España. Miembro GPI AEPap.
ePediatra. CS Virgen Peregrina. Pontevedra. España. Miembro GPI AEPap.
fServicio de Pediatría. Hospital de Riotinto. Huelva. España. Miembro GPI AEPap.
gPediatra.CS La Palomera. León. España. Miembro GPI AEPap.
hPediatra. CS Puente Genil. Córdoba. España. Miembro GPI AEPap.
Correspondence: MR Albañil. E-mail: mralba100@hotmail.com
Reference of this article: Albañil Ballesteros MR, Cubero Santos A, Martínez Chamorro MJ, Muñoz Hiraldo ME, Ares Álvarez J, Morillo Gutiérrez B, et al. Aetiology and antibiotic resistance in paediatric urinary tract infection. A multicentre study in primary care . Rev Pediatr Aten Primaria. 2024;26:361-72. https://doi.org/10.60147/5d2a9a33
Published in Internet: 13-11-2024 - Visits: 12195
Abstract
Introduction: empirical treatment of urinary tract infection (UTI) requires knowledge of antibiotic resistance in the most prevalent uropathogens. The aim of the study was to analyse the pathogens involved in community-acquired paediatric UTIs, their drug resistance profile and the association between resistance and the studied variables.
Patients and methods: nationwide, multicentre, prospective, longitudinal and descriptive study carried out in primary care paediatrics clinics. We included all UTI episodes identified in patients aged 0-15 years in the caseloads of 187 collaborating paediatricians (187 058 patients), regardless of the setting where they were diagnosed or treated, between 1/10/2019 and 31/12/2020.
Results: there were 588 identified UTI episodes. Escherichia coli was the most frequently isolated uropathogen (79.67%), followed by Proteus spp., Klebsiella spp. and Enterococcus spp. Extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae were isolated in 1.52% of episodes. The lowest prevalences of resistance to oral antibiotics corresponded to third-generation cephalosporins, cefuroxime and fosfomycin (3.88%, 4.81% and 6.30%, respectively). The proportions of resistance to first-generation cephalosporins, gentamicin and amikacin were 8.69%, 7.55% and 3.23% respectively. The prevalence of resistance was 23.85% for amoxicillin-clavulanic acid (34.51% in males) and 23.40% for cotrimoxazole.
Conclusions: E. coli was the most frequently isolated uropathogen. Amoxicillin-clavulanic acid and cotrimoxazole should not be used empirically due to the high prevalence of resistance. Second and third generation cephalosporins and fosfomycin could be adequate empirical therapy depending on age and the type of infection. Local susceptibility to first-generation cephalosporins should be tested.
Keywords
● Antibiotics resistance ● antimicrobial stewardship programmes ● Urinary tract infectionINTRODUCTION
Urinary tract infection (UTI) is defined as microbial growth in the urinary tract accompanied by compatible clinical manifestations.1 It is one of the most common types of bacterial infections in the paediatric age group. Approximately 2% of boys and 8% of girls experience at least one episode of UTI in the first 7 years of life.2 The incidence is higher in male infants under 6 months and girls from age 1 year.1-4 The rates of recurrence, which are particularly high in infants under 1 year and patients with urinary tract malformations, reach 10 to 15% in the first 6 to 12 months after the initial episode.
Besides age and sex, there are other risk factors that include renal or congenital anomalies of the kidney or urinary tract (CAKUT), diseases that affect urine flow, vesicoureteral reflux, phimosis in male infants, labial adhesion in girls, urinary dysfunction, constipation, neurogenic bladder, urinary tract catheterization, nephroureterolithiasis and sexual activity in female adolescents.1,3-5 The diagnosis of UTIs is not free of controversy. There is no consensus for the colony-forming unit (CFU) threshold considered diagnostic of UTI. Although in our study we adhered to the criteria published in the document Recomendaciones sobre el diagnóstico y tratamiento de la infección urinaria (Recommendations on the diagnosis and treatment of urinary tract infection),6 other, more recent documents lower the threshold in case of “high likelihood” of a true UTI,1,7 introducing a subjective component. There is consensus that urine bag specimens are not appropriate for urine culture.
Urinary tract infections are usually managed with empiric therapy before the pathogen and its antimicrobial susceptibility profile are known. For this reason, it is crucial to update periodically information on drug resistance in prevalence uropathogens involved in community-acquired infections, taking into account variations associated with patient age, sex, fever or risk factors.3 The aim of our study was to identify the causative pathogens involved in paediatric UTI cases in the primary care (PC) setting and analyse their antimicrobial resistance profile.
PATIENTS AND METHODS
We conducted a nationwide multicenter prospective, longitudinal and observational descriptive study in primary care paediatrics (PCP) clinics. We engaged the collaboration of clinicians through an invitation letter distributed to the members of la Asociación Española de Pediatría de Atención Primaria (Spanish Association of Primary Care Pediatrics, AEPap) and contacts in the primary care pediatrician (PEDIAP) mailing list of the RedIRIS network, which amounted to 4009 members and 1020 contacts, respectively, at the time of the mailing.
From 1/10/2019 to 31/12/2020, we followed up a total of 187 paediatric caseloads in 15 autonomous communities (ACs) in Spain. The collaborating partners were directed to record any diagnosed episode of UTI in their caseloads, whether diagnosed in their clinic or any other setting (hospital, urgent care or private health care system).
Madrid and Castilla y León were the ACs with the largest representation (37.22% and 19.44%, respectively), followed by Galicia (6.67%), Canary Islands (6.11%), Aragón (5.57%) and Andalusia (5%). No clinicians from Murcia, Baleares, Ceuta or Melilla participated. All participants worked in the public health care system, and 76.33% were employed in urban settings. The mean number of patients in the caseloads of collaborating paediatricians was of 1039,21 patients (median, 1020), and all their caseloads cumulatively amounted to 187 058 patients.
Inclusion criteria:
The study universe consisted of children aged 0 to 15 years managed at some point of the diagnosis, treatment or followup in a PCP clinic, independently of the setting of diagnosis. We recorded all episodes with a diagnosis of UTI based on the criteria outlined in the document Recomendaciones sobre el diagnóstico and tratamiento de la infección urinaria: compatible clinical manifestations and growth of microorganisms in urine collected with sterile technique, applying the thresholds presented in Table 1.6
| Table 1. Criteria for clinically significant bacteriuria | |
|---|---|
| Type of sample | Colony count (CFU/mL) |
| Suprapubic puncture | Any |
| Urinary catheter | ≥10 000 |
| Spontaneous urine catch | ≥100 000 Consider 10 000-50 000 in the case of a high likelihood of true urinary tract infection (fever + pyuria-bacteriuria or patients with renal disease) |
|
Source: Recomendaciones sobre el diagnóstico y tratamiento de la infección urinaria6. |
|

Exclusion criteria:
Episodes in patients not allocated to the caseloads of the collaborating clinician, in which followup was not possible or for which informed consent (IC) could not be obtained.
The data were anonymized and collected through an online form (Google Forms platform).
Testing of collected samples (urine culture in every case, urine test strip, urinalysis and urine sediment examination) was conducted in primary care centers and microbiology reference laboratories following the protocols of each facility.
We collected data on the following variables: age, sex, presence of fever (axillary temperature >38 °C), risk factors, consumption of antibiotics in the past month, prophylaxis, isolated pathogen and antimicrobial susceptibility results (antibiogram). We considered the following risk factors: vesicoureteral reflux, renal disease, renal malformation, urinary catheterization, bladder dysfunction, constipation, phimosis, labial adhesions, previous history of UTI and hypercalciuria. There is evidence8,9 suggesting potential differences in the etiological agents and prevalence of drug resistance in UTIs in patients with renal or urinary system disease. Therefore, with the aim of assessing for these potential differences in this case series, we made a separate analysis of episodes with risk factors potentially related to kidney and urinary tract disease (group 1); establishing a second group of episodes with presence of risk factors of a different nature (group 2) and a third group without risk factors (group 3).
We analysed and compared the isolated bacteria and the prevalence of resistance to different antimicrobials based on age, sex and the presence of fever and of risk factors.
Statistical analysis
The analysis was performed with the software JASP version 0.18.3, expressing quantitative variables in terms of percentages and the mean and standard deviation (SD). Qualitative data were compared using the χ2 test. In the case of dichotomous variables, we used the Fisher exact test.
Ethical considerations
The study was carried out in adherence to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the Hospital de Fuenlabrada, Madrid (APR 19/03). Patients were included after obtaining the IC of the parents/legal guardians of all patients in addition to the assent of patients aged more than 12 years.
RESULTS
We registered a total of 588 episodes corresponding to 519 patients. In 12 episodes, two pathogens were isolated, one of which was Escherichia coli. Of these 12 episodes, 9 occurred in children aged more than 2 years, 7 in female patients, 8 were afebrile, 4 occurred in patients without risk factors and 10 were the first UTI episode documented in the patient. There were a total of 600 bacterial isolations. During the period under study, 69 of the recorded episodes were recurrent (more than 1 episode in the same patient). Table 2 summarizes the clinical characteristics of the UTI episodes and the patients.
| Table 2. Clinical characteristics of confirmed episodes of UTI and the corresponding patients | |||
|---|---|---|---|
| Episodes of confirmed UTI | Patients with confirmed UTI | ||
| N | N: 588 (%) | N: 519 (%) | |
| Sex | Female | 439 (74.66) | 380 (73.21) |
| Age | <2 years | 189 (32.14) | 176 (33.91) |
|
55 (9.35) | 53 (10.21) | |
|
99 (16.84) | 93 (17.92) | |
|
35 (5.95) | 30 (5.78) | |
| >2 years | 399 (67.86) | 343 (66.09) | |
|
202 (34.35) | 171 (32.95) | |
|
160 (27.21) | 140 (26.98) | |
|
37 (6.29) | 32 (6.17) | |
| Mean age ± SD (months) | 56.34 ± 47.26 | 55.35 ± 47.56 | |
| Continence | Continent | 377 (64.12) | 329 (63.39) |
| Incontinent due to disease | 6 (1.02) | 3 (0.58) | |
| Incontinent due to age | 205 (34.86) | 187 (36.03) | |
| Risk factor | Total | 258 (43.88) | 198 (38.16) |
| VUR | 39 (6.63) | 24 (4.62) | |
| Renal malformation | 36 (6.12) | 28 (5.39) | |
| Previous UTI | 161 (27.38) | 106 (20.42) | |
| Constipation /encopresis | 51 (8.67) | 43 (8.29) | |
| Bladder dysfunction | 27 (4.59) | 13 (2.50) | |
| Urinary catheterization | 4 (0.68) | 1 (0.19) | |
| Labial adhesions | 12 (2.73)* | 10 (2.63)* | |
| Phimosis | 47 (31.97)** | 44 (32.12)** | |
| Hypercalciuria | 3 (0.51) | 2 (0.39) | |
| RF group 1 | 123 (20.92) | 90 (17.34) | |
| RF group 2 | 135 (22.96) | 108 (20.81) | |
| No RF | 330 (56.12) | 321 (61.85) | |
| Fever | 203 (34.52) | ||
| Combined infection | 12 (2.04) | 12 (2.31) | |
| Single episode | 519 (88.27) | 464 (89.40) | |
| Previous prophylaxis | 25 (4.25) | ||
| Antibiotics in previous month | 76 (12.93) | ||
|
*Over total female patients; **Over total male patients. |
|||

Most frequent pathogens
In all analysed groups, E. coli was the most frequent pathogen. In the total sample, children of either sex, children aged more than 2 years and afebrile episodes, the pathogens following in frequency were Proteus spp., Klebsiella spp. and Enterococcus spp.
In patients with no risk factors or group 2 risk factors, the second most frequent pathogen was also Proteus spp.
In children under 2 years, febrile episodes and patients with group 1 risk factors, Klebsiella spp. was the second most frequent pathogen.
Staphylococcus saprophyticus was isolated in 10 episodes. All these episodes were afebrile and occurred in patients aged more than 2 years, 8 occurred in girls, and all occurred in patients without risk factors saved for a mixed infection in a patient with a previous history of UTI, bladder dysfunction, urinary retention and constipation/encopresis. The urine specimens in all these episodes tested negative for nitrites in the urinalysis.
Tables 3 and 4 present the pathogen distributions in the different groups.
| Table 3. Isolates in the overall sample of episodes, by sex and by age | |||||
|---|---|---|---|---|---|
| Isolates in total sample | Isolates by sex N: 598† | Isolates by age N: 600 | |||
| Pathogen | N: 600 | Male | Female | <2 years | >2 years |
| 152 | 446 | 192 | 408 | ||
| Total (%) | Total (%) | Total (%) | Total (%) | Total (%) | |
| E. coli | 478 (79.67) | 107 (70.39) | 369 (82.74)** | 162 (84.38) | 316 (77.45) |
| Proteus spp. | 44 (7.33) | 19 (12.5)* | 25 (5.61) | 3 (1.56) | 41 (10.05)*** |
| Klebsiella spp. | 30 (5) | 8 (5.26) | 22 (4.93) | 16 (8.33)* | 14 (3.43) |
| Enterococcus spp. | 18 (3) | 7 (4.61) | 11 (2.47) | 4 (2.08) | 14 (3.43) |
| S. saprophyticus | 10 (1.67) | 2 (1.32) | 8 (1.79) | 0 (0) | 10 (2.45)* |
| Citrobacter spp. | 6 (1) | 3 (1.97) | 3 (0.67) | 2 (1.04) | 4 (0.98) |
| Other GNB | 9 (1.5) | 5 (3.29)* | 4 (0.91) | 5 (2.60) | 4 (0.98) |
| Other GP cocci | 5 (0.83) | 1 (0.66) | 4 (0.91) | 0 (0) | 5 (1.23) |
|
†The sex was not documented in 2 patients. *p <0.05; **p <0.005; ***p < 0.001. |
|||||

| Table 4. Distribution of pathogens isolated in febrile and afebrile episodes and based on the presence of risk factors | |||||
|---|---|---|---|---|---|
| Pathogen | Isolate distribution based on presence of fever | Isolate distribution based on risk factors | |||
| Afebrile episodes | Febrile episodes | Episodes with group 1 risk factors | Episodes with group 2 risk factors | Episodes without risk factors | |
| N: 393 | N: 207 | N: 128 | N: 138 | N: 334 | |
| Total (%) | Total (%) | Total (%) | Total (%) | Total (%) | |
| E. coli | 298 (75.83) | 180 (86.96)** | 93 (72.66) | 111 (80.43) | 274 (82.04) |
| Proteus spp. | 41 (10.43)** | 3 (1.45) | 6 (4.69) | 12 (8.70) | 26 (7.78) |
| Klebsiella spp. | 17 (4.33) | 13 (6.28) | 11 (8.59)* | 9 (6.52) | 10 (2.99) |
| Enterococcus spp. | 13 (3.31) | 5 (2.42) | 8 (6.25)* | 2 (1.45) | 8 (2.40) |
| S. saprophyticus | 10 (2.54)* | 0 | 1 (0.78) | 0 | 9 (2.69) |
| Citrobacter spp. | 4 (1.01) | 2 (0.97) | 2 (1.56) | 1 (0.72) | 3 (0.90) |
| Other GNB | 6 (1.53) | 3 (1.45) | 4 (3.13)* | 3 (2.17) | 2 (0.60) |
| Other GP cocci | 4 (1.02) | 1 (0.48) | 3 (2.34) | 0 | 2 (0.60) |
|
*p <0.05; **p <0.001. Group 1 risk factors defined as one or more of the following: vesicoureteral reflux, renal disease, renal malformation, urinary catheterization, bladder dysfunction or constipation. Group 2 risk factors defined as one or more of the following: phimosis, adhesions, history of previous UTI or hypercalciuria. GNB: gram-negative bacilli (include: Edwardsiella tarda, Enterobacter asburiae, Haemophilus parainfluenzae, Enterobacter cloacae, Pseudomonas spp.). GP: gram-positive (other GP cocci include: Aerococcus urinae, group B streptococcus, coagulase-negative Staphylococcus). |
|||||

Prevalence of antimicrobial resistance
Tables 5 and 6 and Figure 1 present the proportion of isolates resistant to the antibiotics used most widely for treatment of UTIs in the total episodes, by group and in the episodes involving the most frequent etiological agent (E. coli).
| Table 5. Antibiotic resistance in UTI episodes, overall and by sex and age | |||||
|---|---|---|---|---|---|
| Resistance in total sample | Resistance by sex | Resistance by age | |||
| Antibiotic | N: 600 | Male N: 152† | Female N: 446† | <2 years N: 192 | >2 years N: 408 |
| Antibiograms (% R) | Antibiograms (% R) | Antibiograms (% R) | Antibiograms (% R) | Antibiograms (% R) | |
| Amoxicillin | 517 (46.42%) | 123 (54.47%)* | 392 (43.88%) | 166 (51.20%) | 351 (44.16%) |
| Amoxicillin-clavulanic | 457 (23.85%) | 113 (34.51%)** | 343 (20.41%) | 148 (28.38%) | 309 (21.68%) |
| Piper-tazobactam | 94 (4.26%) | 26 (7.69%) | 68 (2.94%) | 31 (0%) | 60 (6.67%) |
| Cephalothin/Cefazoline | 69 (8.69%) | 17 (11.76%) | 52 (7.69%) | 24 (12.5%) | 45 (6.67%) |
| Cefoxitin | 106 (7.55%) | 28 (7.41%) | 78 (7.69%) | 34 (14.71%) | 72 (4.17%) |
| Cefuroxime | 478 (4.81%) | 112 (6.25%) | 364 (4.40%) | 156 (4.49%) | 322 (4.97%) |
| Cefotaxime Ceftriaxone |
258 (3.88%) | 72 (2.78%) | 185 (4.32%) | 96 (3.13%) | 162 (4.32%) |
| Ceftazidime | 154 (3.90%) | 39 (5.13%) | 115 (3.48%) | 58 (3.45%) | 96 (4.17%) |
| Cefepime | 148 (2.70%) | 35 (5.71%) | 112 (1.79%) | 49 (2.04%) | 99 (3.03%) |
| Fosfomycin | 524 (6.30%) | 118 (9.32%) | 404 (5.45%) | 157 (3.82%) | 367 (7.36%) |
| Nitrofurantoin | 478 (8.37%) | 108 (14.81%)* | 368 (6.52%) | 148 (5.41%) | 330 (9.70%) |
| Cotrimoxazole | 513 (23.40%) | 124 (29.84%) | 387 (21.45%) | 160 (25.63%) | 353 (22.38%) |
| Trimethoprim | 44 (18.18%) | 10 (30.00%) | 34 (14.71%) | 18 (22.22%) | 26 (15.38%) |
| Ciprofloxacin Ofloxacin |
314 (9.87%) | 68 (10.29%) | 245 (9.80%) | 97 (6.19%) | 217 (11.52%) |
| Norfloxacin | 160 (13.13%) | 34 (8.82%) | 126 (14.29%) | 46 (10.87%) | 114 (14.04%) |
| Nalidixic acid | 88 (23.86%) | 15 (26.67%) | 72 (23.61%) | 26 (19.23%) | 62 (25.81%) |
| Amikacin | 93 (3.23%) | 23 (4.35%) | 70 (2.86%) | 34 (0%) | 59 (5.08%) |
| Gentamicin | 477 (7.55%) | 121 (10.74%) | 354 (6.50%) | 160 (6.25%) | 317 (8.20%) |
| Vancomycin | 25 (8.00%) | 4 (0%) | 21 (9.52%) | 11 (9.09%) | 14 (7.14%) |
| Meropenem | 128 (2.34%) | 31 (3.23%) | 97 (2.06%) | 48 (0%) | 80 (3.75%) |
| Ticarcilin | 30 (40%) | 7 (42.86%) | 23 (39.13%) | 14 (21.43%) | 16 (56.25%) |
| ESBL | 9 (1.50%) | 3 (2.08%) | 6 (1.40%) | 5 (2.70%) | 4 (1.03%) |
|
†Sex was not documented in 2 patients. *p <0.05; **p <0.005. ESBL: extended-spectrum beta-lactamase-producing strain; R: resistant. |
|||||

| Table 6. Antibiotic resistance based on the presence of fever and risk factors | |||||
|---|---|---|---|---|---|
| Resistance based on presence of fever | Resistance based on risk factors | ||||
| Antibiotics | Isolation in febrile episodes N: 207 |
Isolation in afebrile episodes N: 393 |
Isolation in episodes with group 1 risk factors N: 128 |
Isolation in episodes with group 2 risk factors N: 138 |
Isolation in episodes without risk factors N: 334 |
| No. of antibiograms (% R) | No. of antibiograms (% R) | No. of antibiograms (% R) | No. of antibiograms (% R) | No. of antibiograms (% R) | |
| Amoxicillin | 179 (47.49%) | 338 (45.86%) | 110 (57.27%)* | 120 (37.50%) | 287 (45.99%) |
| Amox-clavulanic | 157 (26.11%) | 300 (22.67%) | 97 (26.80%) | 104 (22.12%) | 256 (23.44%) |
| Piperacillin-tazobactam | 37 (2.70%) | 57 (5.26%) | 22 (9.09%) | 23 (0%) | 49 (4.08%) |
| Cephalothin/Cefazoline | 31 (6.45%) | 38 (10.53%) | 15 (13.33%) | 14 (14.29%) | 40 (5.00%) |
| Cefoxitin | 36 (13.89%) | 70 (4.29%) | 18 (11.11%) | 26 (7.69%) | 62 (6.45%) |
| Cefuroxime | 167 (5.99%) | 311 (4.18%) | 97 (9.28%)* | 110 (2.73%) | 271 (4.06%) |
| Cefotaxime Ceftriaxone |
103 (5.83%) | 155 (2.58%) | 52 (5.77%) | 58 (3.45%) | 148 (3.38%) |
| Ceftazidime | 66 (7.58%) | 88 (1.14%) | 35 (5.71%) | 36 (0%) | 83 (4.82%) |
| Cefepime | 60 (6.67%)* | 88 (0%) | 30 (6.67%) | 37 (0%) | 81 (2.47%) |
| Fosfomycin | 175 (2.86%) | 349 (8.02%)* | 107 (9.35%) | 118 (2.54%) | 299 (6.69%) |
| Nitrofurantoin | 162 (4.32%) | 316 (10.44%)* | 91 (8.79%) | 114 (7.02%) | 273 (8.79%) |
| Cotrimoxazole | 167 (20.96%) | 346 (24.57%) | 101(29.70%) | 116 (17.24%) | 296 (23.65%) |
| Trimethoprim | 23 (21.74%) | 21 (14.29%) | 5 (20.00%) | 11 (9.09%) | 28 (21.43%) |
| Ciprofloxacin Ofloxacin |
111 (7.21%) | 203 (11.33%) | 58 (5.17%) | 81 (13.58%) | 175 (9.71%) |
| Norfloxacin | 55 (14.55%) | 105 (12.39%) | 27 (7.41%) | 42 (19.05%) | 91 (12.09%) |
| Nalidixic acid | 37 (16.22%) | 51 (29.41%) | 12 (25.00%) | 16 (37.50%) | 60 (20.00%) |
| Amikacin | 39 (7.70%) | 54 (0%) | 20 (10.00%) | 21 (0%) | 52 (1.92%) |
| Gentamicin | 168 (7.14%) | 309 (7.77%) | 102 (8.82%) | 113 (7.08%) | 262 (7.25%) |
| Vancomycin | 10 (20%) | 15 (0%) | 5 (20.00%) | 2 (0%) | 18 (5.56%) |
| Meropenem | 55 (5.45%) | 73 (0%) | 31 (6.45%) | 28 (0%) | 69 (1.45%) |
| Ticarcilin | 19 (26.32%) | 11 (63.64%) | 6 (50.00%) | 6 (16.67%) | 18 (44.44%) |
| ESBL | 4 (2.02%) | 5 (1.33%) | 1 (0.82%) | 3 (2.29%) | 5 (1.55%) |
|
*p <0.05. Group 1 risk factors defined as one or more of the following: vesicoureteral reflux, renal disease, renal malformation, urinary catheterization, bladder dysfunction or constipation. Group 2 risk factors defined as one or more of the following: phimosis, adhesions, history of previous UTI or hypercalciuria. ESBL: extended-spectrum beta-lactamase-producing strain; R: resistant. |
|||||

| Figure 1. Antibiograms performed in E. coli isolates. The red line marks the 15% resistance prevalence threshold above which the antibiotic is NOT recommended for empiric treatment. |
|---|
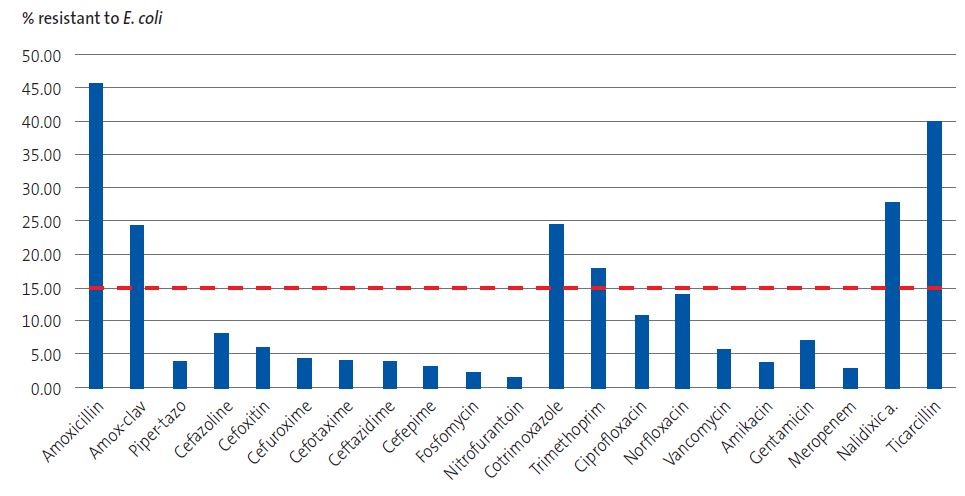 |

In the overall sample of episodes, third-generation cephalosporins, cefuroxime and fosfomycin were the oral antibiotics with the lowest prevalence of resistance (3.88%, 4.81% and 6.30%, respectively).
The prevalence of resistance was 23.85% for amoxicillin-clavulanic acid (34.51% in male patients) and 23.40% for cotrimoxazole. Furthermore, the percentage of resistant isolates was 8.69% for first-generation cephalosporins, 8.37% for nitrofurantoin (14.81% in male patients); 7.55% for gentamicin and 3.23% for amikacin.
In the stratified analysis, we found that the percentages of resistance to amoxicillin, amoxicillin-clavulanic acid and nitrofurantoin were significantly higher in male patients. The percentages of resistance to fosfomycin and to nitrofurantoin were significantly greater in in afebrile episodes, the percentage of resistance to cefepime significantly greater in febrile episodes and the percentages of resistance to cefuroxime and amoxicillin significantly greater in patients with risk factors potentially related to renal disease.
We did not find any significant differences based on age.
Nine isolates (1.5% of episodes) corresponded to extended-spectrum beta-lactamase (ESBL)-producing enterobacteria. Only one of these episodes occurred in a patient currently receiving prophylaxis who had consumed antibiotics in the past month.
In 96 episodes (16.33% of the total), patients reported a history of antibiotic treatment and/or prophylaxis in the past month. In 20 episodes (3.40% of the total), the patient had received antibiotics for prophylaxis, but not for treatment, in the past month. And in 71 episodes (12.07% of the total), the patient had consumed antibiotics for treatment but not for prophylaxis.
We also calculated frequencies of resistance for the different Acs, but due to the small number of episodes in some of the regions we were unable to obtain significant results.
DISCUSSION
The findings of this study carried out in the PC setting, in which 588 episodes of pediatric UTI were analysed, confirmed that E. coli was the leading uropathogen, responsible for nearly 80% of UTI episodes. This was consistent with recent studies conducted in Spain that reported percentages of 80.75%10 and 76.9%11, respectively, exceeding those of previous studies (58.9-64%).12-15 This increase could be associated with the greater percentage of specimens collected at the PC level in our series (65.40%) compared to other studies (53%13 or 55.1%12). The causative pathogens that followed in frequency, Proteus spp. and Klebsiella spp., were consistent with other studies conducted in Spain.10-12
It should be noted that while E. coli was the most frequent uropathogen in all the groups considered in the analysis, there was a percentage of UTI episodes, which varied between groups and was almost as high as 30% in patients of male sex or with group 1 risk factors, in which the causative agent was a different pathogen.
In our study, we did not take into account the location of the UTI (upper vs. lower urinary tract), but we did consider the presence of fever (febrile vs. afebrile episodes). In agreement with other case series,10 most episodes of febrile UTI were caused by E. coli (86.96%), followed by Klebsiella spp., with frequencies that far exceeded those of other pathogens.
E. coli was isolated in 72.66% of episodes occurring in patients with group 1 risk factors, followed in frequency by Klebsiella spp. (8.59%), Enterococcus spp. (6.25%) and Proteus spp. (4.69%). These findings were consistent with the distribution published by Chamorro et al.8 and differed from those of the series published by Oltra-Benavent et al., in which the proportion of episodes caused by microorganisms other than E. coli was greater (46.8%).9 Both series consisted of patients with pyelonephritis and renal or urologic disease, although in the latter, 25.5% of the episodes occurred in patients with a history of urologic surgery.
We did not find significant differences in the isolates between boys with and without phimosis.
S. saprophyticus accounted for 1.67% of isolates. Given its infrequent nitrite formation and more frequent involvement in sexually active female adolescents,16 we propose considering performance of urine culture in this group when a UTI is suspected, even in the case of nitrite-negative results in the dipstick test.
In 2.04% of episodes, two pathogens were isolated from culture. Although the presence of 2 pathogens in a urine culture suggests contamination,4,5 based on the literature, it is recommended that clinical features are taken into account to decide whether these results should be interpreted as a true UTI: complicated versus uncomplicated UTI, sample collection method, type of isolate (uropathogen or saprophyte), presence of pyuria, etc.5,17 The frequency of mixed infection in our study was slightly larger than the frequency reported in another study conducted in Spain.11
The resistance rates for amoxicillin-clavulanic acid and cotrimoxazole were very high (23.85% and 23.40%), so these drugs should not be used for empiric therapy unless there is evidence of a lower prevalence of resistance at the local level. The best sensitivity profiles corresponded to cefuroxime, cefixime and fosfomycin, which therefore could be (depending on the age and type of infection) the first-line antibiotics while awaiting the results of the antibiogram.
Our findings regarding the prevalence of isolates resistant to different antibiotics were consistent with or, in some cases, exceeded the previously reported figures.11-16,18. It is worth noting the high prevalence of resistance to amoxicillin-clavulanic acid, which exceeded 30% in some groups, probably due to its widespread use on account of either inappropriate prescription or shortages of antibiotics with a narrower spectrum. This percentage would preclude its use for empiric treatment of UTIs based on the recommendation to avoid antibiotics exceeding the resistance prevalence threshold of 15%.1 Based on our findings, amoxicillin, amoxicillin-clavulanic acid, cotrimoxazole, trimethoprim and nalidixic acid should not be used for empiric treatment of UTI.
Treatment options for UTI are currently limited by recurrent shortages in cefuroxime axetil and restrictions for the use of fosfomycin, which has a good susceptibility profile but is only recommended from age 12 years,19 and nitrofurantoin (which is only approved by the Spanish Agency of Medicines and Medical Devices [AEMPS] for treatment of uncomplicated cystitis in female patients aged more than 3 months).20 As a result, in children aged less than 12 years, third-generation cephalosporins become the default first-line agent for empiric treatment, even in the case of lower UTI.
In this context, we ought to highlight the rates of resistance to first-generation cephalosporins observed in our study, which were lower compared to the previous literature,13,18 although the low frequency with which these agents were included in the antibiogram (67 compared to 474 for second-generation cephalosporins) calls for caution and evinces the need to perform further studies to obtain local prevalence data to guide the prescription of empiric antibiotherapy, as recommended in the Clinical Practice Guideline recently published in Spain.21
We also found a lower prevalence of resistance to amikacin (3.33%) compared to gentamicin (7.55%), probably due to the more restricted use of the former.
When it came to quinolones, we found a significant prevalence of resistance despite their exceptional use in paediatric patients.
A particular trend in the broader problem of antimicrobial drug resistance is the increase in the frequency of community-acquired UTIs caused by ESBL-producing E. coli. Some studies have found an increase of up to 3.54% in recent years, corresponding to an annual increase of 0.51%.22 The increase observed in our study (1.50%) is consistent with previously published data for Spain,12-14,18 although frequencies as high as 17% have been reported in series with a large proportion of patients with renal or urologic disease.9,23. Of the 9 such isolates in our study, only one corresponded to a patient with renal or urologic disease.
With regard to the prevalences of drug resistance in the different CAs, we observed variations, but the small number of samples in some of these regions limits the generalizability of the results.
There are limitations to our study: the data obtained may not be representative of all the ACs due to uneven participation. Of a total of 404 clinicians that initially agreed to collaborate, only 187 were able to participate in the study. This was partly due to administrative barriers, a common hurdle in multicenter studies,24 despite the observational design of the project.
There was variation in the number of antibiograms that included each of the antibiotics, although the numbers can be considered sufficient for the antibiotics used most frequently for treatment of community-acquired infections.
A large portion of the study period coincided with the COVID-19 pandemic. The state of alert was declared 5 months after the start of followup and changes in health care could have affected data collection, the management of the episodes and the total number of UTIs diagnosed appropriately. In the final months of the study, UTI episodes may have been underrecorded on account of the excessive burden placed on the health care system by the resumption of in-person care delivery, concurrent to the different waves of the pandemic.
Although staying up to date with local drug resistance data is recommended, in practice it is very difficult to have a sufficient number of pediatric cultures in an area close to the patient. It is more common for reported data to be aggregated and not broken down by age or origin of the specimens (community vs hospital). For this reason, we believe that the data collected in our study, despite its limitations, are relevant for the practice of primary care paediatricians.
CONCLUSION
To conclude, the most prevalent uropathogen causing UTI continues to be E. coli, although in certain groups it may be necessary to consider other bacteria. Amoxicillin, amoxicillin-clavulanic acid, cotrimoxazole, trimethoprim and nalidixic acid should not be used for empiric treatment. Local patterns of sensitivity should be evaluated for first-generation cephalosporins, which are currently not considered for first-line treatment.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article. The study was awarded a grant for Research in Primary Care from the Asociación Española de Pediatría de Atención Primaria - Fundación para la Salud (AEPap-FPS) in 2019.
AUTHORSHIP
Author contributions: drafting of manuscript (MRAB, ACS), statistical analysis of the data (RJA), study design, coordination of collaborating partners, literature search and final revision of the manuscript (all).
Partial preliminary data from this study were presented as a communication at the 19th Congreso de Actualización en Pediatría (Paediatrics Update Congress) of the AEPap in 2023.
ABBREVIATIONS
AC: autonomous community · CFU: colony-forming unit · ESBL: extended spectrum beta-lactamase · GNB: gram-negative bacilli · GP: gram-positive · IC informed consent · PC: primary care · PCP: primary care paediatrics · SD: standard deviation · UTI: urinary tract infection.
ACKNOWLEDGMENTS
We thank all professionals that collaborated in the identification and recording of cases.
REFERENCES
- González Rodríguez JD, Justa Roldán MJ. Infección de las vías urinarias en la infancia. Protoc diagn ter pediatr. 2022;1:103-29.
- Redondo Sánchez J, Domínguez Lázaro AM, Rodríguez Barrientos R, Barrio Cortes J, Seoane Sanz J, Bravo Acuña JJ, et al. Tendencias en la hospitalización por infección del tracto urinario en la población pediátrica de España en el período 2000-2015. An Pediatr (Barc). 2023;98:175-84. https://doi.org/10.1016/j.anpede.2023.01.009
- Tullus K, Shaikh N. Urinary tract infections in children. Lancet. 2020;395:1659-68. https://doi.org/10.1016/s0140-6736(20)30676-0
- Mattoo TK, Shaikh N, Nelson CP. Contemporary Management of Urinary Tract Infection in Children. Pediatrics. 2021;147:e2020012138. https://doi.org/10.1542/peds.2020-012138
- Buettcher M, Trueck J, Niederer-Loher A, Heininger U, Agyeman P, Asner S, et al. Swiss consensus recommendations on urinary tract infections in children. Eur J Pediatr. 2021;180:663-74. https://doi.org/10.1007/s00431-020-03714-4
- Piñeiro Pérez R, Cilleruelo Ortega MJ, Ares Álvarez J, Baquero Artigao F, Silva Rico JC, Velasco Zúñiga R, et al. Recomendaciones sobre el diagnóstico y tratamiento de la infección urinaria. An Pediatr(Barc). 2019;90:400.e1-e9. https://doi.org/10.1016/j.anpedi.2019.02.009
- Brandström P, Lindén M. How Swedish guidelines on urinary tract infections in children compare to Canadian, American and European guidelines. Acta Paediatr. 2021;110:1759-71. https://doi.org/10.1111/apa.15727
- Chamorro Sanz N, Ferreiro-Mazón García-Plata P, García Espinosa l, Ruíz Domínguez JA, Molina Gutiérrez MA. Infección urinaria febril en el niño con patología nefrourológica. An Pediatr (Barc). 2021;95:391-2. http://doi.org/10.1016/j.anpedi.2021.03.011
- Oltra Benavent M, Ferrer Lorente B, Ródenas Moreno M, Torrejón Rodríguez l. Selección del tratamiento antibiótico empírico en pielonefritis según el perfil del paciente [Selection of empirical antibiotic treatment in pyelonephritis according to the patient's profile]. An Pediatr (Engl Ed). 2020;92:181-2. http://doi.org/10.1016/j.anpedi.2019.09.012
- Martínez Campos l, Carazo Gallego B, Berghezan Suárez A, Álvarez Ares J, Cruz Cañete M, Olmedo Díaz I. Características clínicas y microbiológicas de una muestra de 1200 urocultivos pediátricos. In: Asociación Española de Pediatría. Libro de ponencias y Comunicaciones 67 Congreso AEP (p. 551-552) [online] [accessed 05/11/2024]. Available at congresoaep.org/static/upload/ow28/events/ev231/Site/files/libro/551/
- Vázquez Pérez A, Alonso Acero l, Baquero Artigao F, De Pablos Gómez M, Calvo C. Infección urinaria comunitaria: etiología, resistencias y perfil del paciente en un hospital de referencia. An Pediatr(Barc). 2022;96:77-80. https://doi.org/10.1016/j.anpedi.2021.06.015
- Rodríguez Lozano J, De Malet A, Cano ME, De la Rubia l, Wallmann R, Martínez Martínez l, et al. Antimicrobial susceptibility of microorganisms that cause urinary tract infections in pediatric patients. Enferm Infecc Microbiol Clin. 2018;36:417-22. https://doi.org/10.1016/j.eimc.2017.08.003
- Moya Dionisio V, Díaz Zabala M, Ibáñez Fernández A, Suárez Leiva P, Martínez Suárez V, Ordóñez Álvarez FA, et al. Patrón de aislamiento bacteriano y sensibilidad antimicrobiana en urocultivos positivos obtenidos de una población pediátrica. Rev Esp Quimioter. 2016;29:146-50.
- Artero López J, Gutiérrez Soto B, Expósito Ruiz M. Etiología de las infecciones urinarias en nuestra Área Sanitaria y perfil de sensibilidad de los uropatógenos más frecuentes. Arch Esp Urol. 2021;74:197-207.
- Sorlózano Puerto A, Gómez Luque JM, Luna del Castillo JD, Navarro Marí JM, Gutiérrez Fernández J. Etiological and Resistance Profile of Bacteria Involved in Urinary Tract Infections in Young Children. Biomed Res Int. 2017;2017:4909452. https://doi.org/10.1155/2017/4909452
- Lo DS, Shieh HH, Barreira ER, Ragazzi SL, Gilio AE. High Frequency of Staphylococcus Saprophyticus Urinary Tract Infections Among Female Adolescents. Pediatr Infect Dis J. 2015;34:1023-5. https://doi.org/10.1097/INF.0000000000000780
- Zboromyrska Y, De Cueto López M, Alonso Tarrés C, Sánchez Hellín V. 2019. 14b. Diagnóstico microbiológico de las infecciones del tracto urinario. Zboromyrska Y (coord.). Procedimientos en Microbiología Clínica. Cercenado Mansilla E, Cantón Moreno R (eds.). Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica. (SEIMC). 2019 [online] [accessed 05/11/2024]. Available at https://seimc.org/contenidos/documentoscientificos/procedimientosmicrobiologia/seimc-procedimiento14a.pdf
- Martínez Campos l, Carazo Gallego B, Piñeiro Pérez R, Méndez Hernández M, Conejo Fernández A. Perfil de sensibilidad a antimicrobianos de las bacterias causantes de infección urinaria de la comunidad en la población pediátrica española. In: Asociación Española de Pediatría. Libro de ponencias y Comunicaciones 67 Congreso AEP (p. 616-617) [online] [accessed 05/11/2024]. Available at www.congresoaep.org/static/upload/ow28/events/ev231/Site/files/libro/616/
- Recomendaciones para restringir el uso de los antibióticos con fosfomicina (EMA/317719/2020). In: Agencia Europa de Medicamentos (EMA) [online] [accessed 05/11/2024]. Available at www.ema.europa.eu/en/documents/referral/fosfomycin-article-31-referral-recommendations-restrict-use-fosfomycin-antibiotics_es.pdf
- Nitrofurantoína (FURANTOÍNA®): nuevas restricciones de uso. In: Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Ministerio de Sanidad, Asuntos Sociales e Igualdad [online] [accessed 05/11/2024]. Available at www.aemps.gob.es/informa/notasInformativas/medicamentosUsoHumano/seguridad/2016/docs/NI-MUH_FV_16-nitrofurantoina.pdf
- Grupo de trabajo de la Guía de Práctica Clínica sobre Infección del Tracto Urinario en la Población Pediátrica. Guía de Práctica Clínica. Infección del Tracto Urinario en la Población Pediátrica. Actualización 2024 [online] [accessed 05/11/2024]. Available at https://serviciopediatria.com/wp-content/uploads/2024/05/2024_GPC-Infeccion-Tracto-Urinario-en-Pediatria.pdf
- Collingwood JD, Yarbrough AH, Boppana SB, Dangle PP. Increasing Prevalence of Pediatric Community-acquired UTI by Extended Spectrum β-Lactamase-producing E. coli: Cause for Concern. Pediatr Infect Dis J. 2023;42:106-9. https://doi.org/10.1097/INF.0000000000003777
- Pérez Heras I, Sanchez Gomez JC, Beneyto Martin P, Ruano de Pablo l, Losada Pinedo B. Community-onset extended-spectrum β-lactamase producing Escherichia coli in urinary tract infections in children from 2015 to 2016: Prevalence, risk factors, and resistances. Medicine (Baltimore). 2017;96:e8571. https://doi.org/10.1097/MD.0000000000008571
- Balaguer Martínez JV, Ciriza Barea E, Carballal Mariño M, García Vera C; Grupo de Investigación y Red de Investigación en Pediatría de Atención Primaria (PAPenRed) de la Asociación Española de Pediatría de Atención Primaria (AEPap). Research in pediatrics: A long bureaucratic obstacle course. An Pediatr (Engl Ed). 2023;99:153-4. https://doi.org/10.1016/j.anpede.2023.06.017





