Vol. 24 - Num. 95
Original Papers
Mycoplasma pneumoniae: differential clinical features and diagnostic challenges in atypical pneumonias in children
Mercedes Cemeli Canoa, Mónica López Camposb, Pilar Oliván Otalc, Eva M.ª Navarro Serranod, M.ª Isabel Lostal Graciab, César García Verae
aPediatra. CS Valdespartera. Zaragoza. España.
bPediatra. CS Actur Norte. Zaragoza. España.
cCS Canal Imperial. Zaragoza. España.
dPediatra. CS Alfajarín. Zaragoza. España.
ePediatra. CS José Ramon Muñoz Fernández. Zaragoza. España.
Correspondence: M Cemeli. E-mail: cano.mcemeli@salud.aragon.es
Reference of this article: Cemeli Cano M, López Campos M, Oliván Otal P, Navarro Serrano EM, Lostal Gracia MI, García Vera C. Mycoplasma pneumoniae: differential clinical features and diagnostic challenges in atypical pneumonias in children . Rev Pediatr Aten Primaria. 2022;24:273-84.
Published in Internet: 11-10-2022 - Visits: 24576
Abstract
Introduction: pneumonia caused by Mycoplasma pneumoniae continues to be underdiagnosed in paediatric primary care, especially in younger children.
Material and methods: prospective study conducted in 9 primary care paediatric caseloads, including children aged 1 month to 14 years with pneumonia diagnosed based on compatible radiographic findings and clinical features. The aetiological diagnosis was made by acute-phase serological testing. We analysed the association of different variables with atypical and typical aetiologies.
Results: of the 92 patients in the sample, 30.4% had atypical pneumonias which, while not rare in children under 2 years (26%) predominated in children over 5 years (80.9%). The mean age in months was significantly higher in cases with an atypical (74.2 ± 42.2) versus typical (35.9 ± 33.8) aetiology (p <0.0001). Nasal congestion was significantly more frequent in non-atypical cases (42.8%; OR 1.8; p <0.01) and tachypnoea in atypical cases (64.2%; OR 2.4; p <0.014). The alveolar pattern was observed in 53.6% of atypical pneumonias, with no differences compared to typical pneumonias. Only 25% of atypical pneumonia cases were treated correctly with first-line macrolide monotherapy, with no differences in outcomes based on the choice of antibiotherapy. Patients with typical pneumonia required intravenous antibiotic therapy more frequently (15.6%), but the difference was not statistically significant.
Conclusion: atypical germs were more frequent at younger ages than previously described, in some cases with concomitant detection of viruses. Improving the diagnosis and treatment of atypical pneumonia remains a challenge.
Keywords
● Mycoplasma pneumoniae ● Pneumonia ● Primary careINTRODUCTION
Community-acquired pneumonia (CAP) is a frequent reason for primary care visits, and in most cases its aetiological diagnosis poses a significant challenge.1-3 In the past decade, changes in epidemiological trends show that the clinical and radiographic characteristics defined traditionally do not always help achieve an accurate diagnosis in clinical practice, as evinced by different studies.4-7 Even in the hospital setting, microbiological tests are not sensitive or specific enough, and the aetiology of pneumonia can only be established in 40-80% of cases.8
The main aetiological agents are believed to be viral and pneumococcal, with viruses involved mainly in children under 5 years and pneumococci in children of any age. In the past 15 years, there has been an increase in the incidence of cases caused by atypical viruses and bacteria, which may account for up to 30% of cases of CAP. Thus, depending on the signs and symptoms, it may be difficult to differentiate between typical and atypical pneumonia.
Therefore, knowledge of current epidemiological trends is important in order to revise and improve guidelines for the management of pneumonia in paediatric practice. In this study, we analysed the characteristics of cases of pneumonia of atypical aetiology compared to all other cases to guide the management of this disease in paediatric patients at the primary care level.
MATERIAL AND METHODS
We conducted a prospective observational and analytical study in 9 primary care paediatric caseloads in the province of Zaragoza, Spain, including children aged 1 month to 14 years with pneumonia diagnosed based on compatible radiographic findings and clinical features in a 2-year period. The anteroposterior chest X-ray was considered the gold standard for diagnosis of CAP.
The case definition of pneumonia was the presence in a previously healthy patient of compatible respiratory symptoms associated with an alveolar, interstitial, mixed or undefined radiographic pattern.
We excluded patients aged less than 1 month and more than 14 years, with primary or secondary immunodeficiency, oncological disease, lung disease (cystic fibrosis, poorly controlled asthma, lung sequestration, bronchiectasis, bronchopulmonary dysplasia), encephalopathy with a risk of bronchoaspiration or with a positive Mantoux test, cases of pneumonia in children that were hospitalised 7 to 14 days before the onset of symptoms or who had the onset in the first 48 hours of the hospital stay, patients that received a diagnosis of pneumonia from a clinician other than the one usually involved in the study or who had received the diagnosis in a different department with a time interval greater than 5 days, and patients for whom we were unable to obtain informed consent.
The primary outcome was the type of pneumonia (atypical vs non-atypical, including pneumococcal and viral cases), and the secondary outcomes included demographic variables (age, sex) epidemiological variables (history of bronchitis and/or pneumonia, vaccination), clinical variables (temperature, cough, respiratory rate [RR] applying the World Health Organization [WHO] criteria,9 shortness of breath, oxygen saturation and other extrapulmonary manifestations), radiological features, laboratory features (white blood cell count, neutrophils, C reactive protein [CPR], IgM antibodies against Mycoplasma pneumoniae) and treatment-related variables.
The diagnosis of atypical pneumonia was based on serologic testing for detection of M. pneumoniae (enzyme-linked immunoassay [ELISA] technique based on the reaction of antibodies in the sample with the antigen bound to the polystyrene surface), which can quantify IgM with a sensitivity of 81-89% in children.10,11
The case definition of non-atypical pneumonia included cases of probable pneumococcal aetiology (not meeting the criteria for other types of pneumonia), mixed aetiology (simultaneous detection of more than 2 pathogens) and cases of viral aetiology diagnosed based on positive results of viral panel testing (respiratory syncytial virus [RSV], influenza A and B, parainfluenza 1, 2, and 3, metapneumovirus and adenovirus) in nasopharyngeal aspirate (NPA) samples.
All collected data were handled in adherence to Organic Law 15/99 on the protection of personal data. The study database did not include any personal identifiable information. Each patient was assigned a code known only to the research team. The study was approved by the Research Ethics Committee of the Autonomous Community of Aragon (CEICA) on February 17, 2017, under file C.P.C.I. PI17/0000, minute no. 02/2017.
Statistical analysis
We conducted a descriptive analysis, summarising quantitative variables with measures of central tendency and dispersion (mean, median and standard deviation) and qualitative variables with absolute frequencies and percentages with the corresponding 95% confidence intervals (CIs). In the inferential analysis, we assessed the association between variables by calculating odds ratios (ORs) and mean differences (MDs) with the corresponding 95% CIs. We assessed the statistical significance by means of the chi square test with the Yates correction or the Fisher exact and Student t tests. If any of the distributions were not normal in the mean comparison test, we used the nonparametric Mann-Whitney U test. We performed univariate analyses to assess the association of clinical, laboratory and radiological variables with the potential aetiology. We also fitted a multivariate logistic regression model to predict the type of pneumonia based on different variables, including those that appeared to be associated with the type of pneumonia based on the results of the univariate analysis (if p <0.10) or biological plausibility. For all tests, we defined statistical significance as p ≤0.05.
Lastly, we analysed the agreement between the initial diagnoses of suspected atypical pneumonia made by collaborating clinicians based on clinical and radiographic features and the diagnoses of atypical pneumonia based on positive serological test results. We excluded cases with an initial diagnosis of undefined or mixed pneumonia to facilitate the concordance analysis.
RESULTS
The study included a total of 92 patients, 30.4% (95% CI: 21.9 to 40.5%) with a diagnosis of atypical pneumonia caused by M. pneumoniae and 69.6% with non-atypical forms of pneumonia. Of the patients with atypical pneumonia, 64.3% (95% CI: 45.8 to 79.3%) were female, compared to 48.4% (95% CI: 36.6 to 60.4%) of patients with non-atypical pneumonia, a difference that was not statistically significant.
Samples for serologic testing for detection of M. pneumoniae could be obtained in 81 patients. High titres of IgM antibodies against M. pneumoniae were detected in 39.5%. Of these cases, 14.2% were mixed infections by M. pneumoniae and a virus (chiefly RSV). When it came to serological testing for atypical pathogens, one patient was found to have a mixed infection by M. pneumoniae and Chlamydia pneumoniae, and another patient tested triple-positive for M. pneumoniae, cytomegalovirus and parvovirus B19.
Serologic tests were chiefly positive in children aged more than 5 years (80.9%; 95% CI: 60 to 92.3%), but accounted for only 26% of cases in children under 3 years (95% CI: 12.5 to 46.5%) (Table 1).
| Table 1. Frequency of cases with a positive serologic test for detection of IgM against M. pneumoniae by age group | ||||||||
|---|---|---|---|---|---|---|---|---|
| M. pneumoniae serology | ≤24 months | 25-60 months | >60 months | Total | ||||
| N | % | N | % | N | % | N | % | |
| Positive | 6 | 26.09 | 9 | 24.33 | 17 | 80.95 | 32 | 39.51 |
| Negative | 17 | 73.91 | 28 | 75.67 | 4 | 19.05 | 49 | 60.49 |
| Total | 23 | 28.39 | 37 | 45.68 | 21 | 25.93 | 81 | 100 |

The mean age in months was significantly higher in cases of atypical pneumonia (74.2 ± 42.2 months) compared to the rest (35.9 ± 33.8 months); MD: 38.32 (95% CI: 20.11 to 56.54; p <0.001).
November and April (each of which accounted for 17.5% of atypical cases of pneumonias) were the months with the highest incidence of atypical pneumonia, although the difference compared to other months was not statistically significant.
Bronchitis was the most frequent relevant feature of the past history, and was more frequent in cases of non-atypical pneumonia (26.56%) compared to typical pneumonia (17.8 %), without significant differences, which was also the case in the analysis of the past history of pneumonia.
As can be seen in Table 2, there were no significant differences in the clinical presentation and certain signs between atypical and non-atypical pneumonia. Fever above 38 °C was less frequent in atypical pneumonia cases, however, in the subset of cases presenting without fever (n = 5), 60% were of atypical pneumonia. Severe cough occurred in a higher proportion of patients with atypical pneumonia, but was absent in 3.6% of atypical cases. Other features that were not significantly associated with atypical pneumonia were gastrointestinal symptoms, conjunctivitis, general malaise, pain in the ribs, painful swallowing, cutaneous exanthema and hypoxaemia with a Sat O2 <92%, although they occurred in a lesser proportion compared to non-atypical pneumonia cases. In contrast, nasal congestion was significantly more frequent in non-atypical cases (42.8%; OR: 1.8; p <0.01) and tachypnoea in atypical cases (64.2%; OR: 2.4; p = 0.0144). Most patients did not exhibit shortness of breath, a feature that was only found in 7.1% of cases due to M. pneumoniae. We ought to highlight that out of all patients who experienced rib pain, 60% received a diagnosis of atypical pneumonia and 40% of non-atypical pneumonia.
| Table 2. Distribution of clinical characteristics in atypical and non-atypical pneumonia | |||
|---|---|---|---|
| Clinical characteristics | Atypical pneumonia n = 28 |
Non-atypical pneumonia n = 64 |
p value |
| n (%) | n (%) | ||
| Feber ≥38 °C | 23 (82.14) | 59 (92.18) | 0.2890 |
| Severe cough | 20 (71.42) | 43 (67.18) | 0.8736 |
| Gastrointestinal symptoms | 4 (14.28) | 13 (20.31) | 0.6940 |
| Rib pain | 3 (10.71) | 2 (3.12) | 0.1631 |
| Nasal congestion | 12 (42.85) | 51 (79.68) | <0.001 |
| Cutaneous exanthema | 1 (3.57) | 2 (3.12) | 0.9911 |
| Odynophagia | 2 (7.14) | 3 (4.68) | 0.9983 |
| Conjunctivitis | 0 (0) | 4 (6.25) | 0.3101 |
| General malaise | 5 (17.85) | 13 (20.31) | 0.7847 |
| Tachypnoea | 18 (64.28) | 20 (31.25) | 0.0144 |
| Hypoxaemia: saturation de O2 ≤92% | 3 (10.71) | 7 (10.93) | 0.9740 |

Figure 1 shows the lack of significant differences between the types of pneumonia in the radiographic patterns described above and in the presence of pleural effusion. A single case caused by M. pneumoniae could not be classified into any of the radiographic patterns by the researcher. Thus, we ought to highlight that the alveolar pattern was found in a percentage of cases of atypical pneumonia that was very close to the one found in the other group, while the interstitial pattern was more frequent in atypical pneumonia compared to non-atypical pneumonia, although the difference was not statistically significant (p = 0.5042). Pleural effusion was slightly more frequent in atypical pneumonia compared to non-atypical pneumonia, although not significantly so (p= 0.9890).
| Figure 1. Percent distribution of radiographic patterns and pleural effusion in atypical and non-atypical pneumonia cases (n = 91) |
|---|
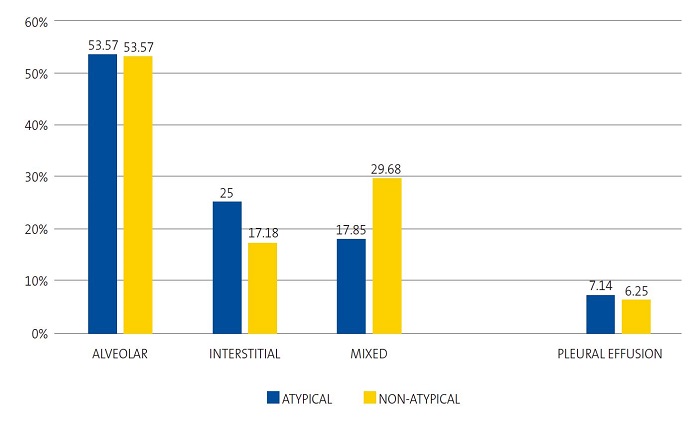 |

Table 3 presents the laboratory test values and their comparison. The white blood cell and neutrophil counts were slightly higher in non-atypical pneumonia, but the difference was not statistically significant. None of the patients with infection by M. pneumoniae had CRP levels >6 mg/dl. The CPR levels were higher in the non-atypical pneumonia group (6.6 ± 10.7 mg/dl) compared to the atypical pneumonia group (1.7 ± 1.7 mg/dl), with a MD of −4.8 (95% CI: −7.6 to −1.9; p = 0.0612).
| Table 3. Distribution of laboratory test results in atypical and non-atypical pneumonia. Quantitative variables expressed as mean ± standard deviation if they followed a normal distribution (white blood cell count), and otherwise as median and interquartile range | ||||
|---|---|---|---|---|
| Atypical pneumonia | Non-atypical pneumonia | MD; 95% CI and statistical significance | ||
| WBC count x 1000 | n = 28 | n = 60 | ||
| Mean | 10 817 | 12 013 | -1196; -3590 to 1199 (p = 0.3220) | |
| Standard deviation | 5015 | 5662 | ||
| Median | 9500 | 10 800 | ||
| Interquartile range | 7400-13 575 | 7875-15 225 | ||
| Mode | 5800 | 10 900 | ||
| Neutrophil count x 1000 | n = 28 | n = 60 | ||
| Mean | 5260 | 6376 | -1116; -3150 to 918 (Mann Whitney U, p = 0.4965)* | |
| Standard deviation | 3992 | 5311 | ||
| Median | 4600 | 4150 | ||
| Interquartile range | 2450-5975 | 2700-8750 | ||
| Mode | 4800 | 2700 | ||
| CRP (mg/dl) | n = 24 | n = 60 | ||
| Mean | 1.79 | 6.59 | -4.80; -7.65 to -1.95 (Mann Whitney U, p = 0.0612)* | |
| Standard deviation | 1.71 | 10.72 | ||
| Median | 0.92 | 2.40 | ||
| Interquartile range | 0.38-2.33 | 0.46-6.28 | ||
| Mode | 0 | 0 | ||

The frequency of admission in non-atypical pneumonia cases (27.2 %; 95% CI: 21.2 to 43.4%) was significantly higher compared to atypical pneumonia (7.1%; 95% CI: 1.9 to 22.6%) (OR: 1.4; 95% CI: 1.1 to 1.7; p = 0.0490), without differences in the reasons for admission.
We found significant differences in the use of amoxicillin (OR: 2.7; 95% CI: 1.1 to 6.9; p = 0.0286) to treat non-atypical compared to atypical pneumonia, and the use of azithromycin (RP: 4.1; 95% CI: 2.7 to 5.8; p = 0.0002) to treat atypical pneumonia compared to all other aetiologies; however, there were no differences in the frequency of combined treatment, which was very similar in non-atypical and atypical pneumonia (OR: 0.4; 95% CI: 0.1 to 1.4; p = 0.3154). Thus, as can be seen in Figure 2, a fourth of cases of atypical pneumonia were correctly treated with azithromycin as monotherapy and 21.42% with combined therapy with amoxicillin and azithromycin, without significant differences in the choice of antibiotherapy (OR: 2.2; 95% CI: 0.6 to 7.3; p = 0.3154). Intravenous antibiotherapy was mainly used in cases of non-atypical pneumonia, a result that was not significant.
| Figure 2. Percent distribution of most frequently used antibiotic agents in atypical and non-atypical pneumonia cases (n = 92) |
|---|
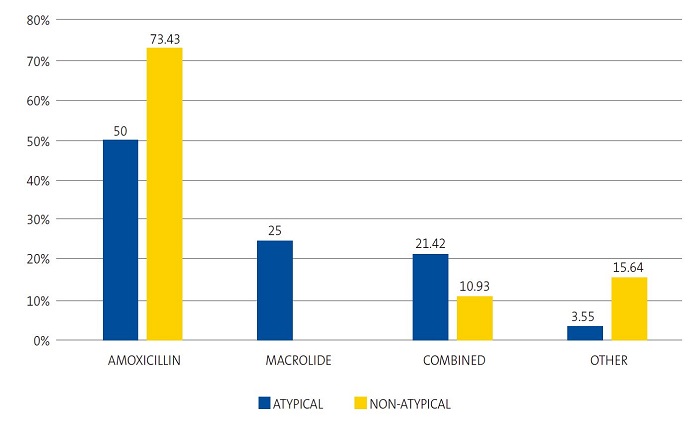 |

In terms of clinical outcomes, a duration of fever > 48 horas was more frequent in non-atypical pneumonia cases (25%) compared to typical pneumonia cases (11.1%), without significant differences. However, at 1 month of treatment, cough persisted more frequently in atypical pneumonia cases (11.1%; 95% CI: 3.8 to 28.1 %; p = 0.3153), although the lung and heart sounds on auscultation normalised earlier in this group (88.9%; 95% CI: 71.9 to 96.1 %; p = 0.2172).
Tables 4 and 5 present the variables that were significantly associated with the type of pneumonia. We also conducted a multivariate analysis comparing the three main types of pneumonia (bacterial with suspected pneumococcal aetiology, atypical bacterial and viral). The mean age was significantly greater in atypical pneumonia compared to suspected pneumococcal pneumonia. Furthermore, the proportions of patients with hypoxemia and admitted to hospital were lower in atypical pneumonia cases compared to all other types. The CRP levels were only significantly higher in cases of viral pneumonia compared to cases of atypical pneumonia.
| Table 4. Summary of main results of the univariate analysis comparing suspected pneumococcal pneumonia and atypical pneumonia. Quantitative variables expressed as mean ± standard deviation if they followed a normal distribution (white blood cell count), and otherwise as median and interquartile range (neutrophils and CRP) | ||||
|---|---|---|---|---|
| Total 92 (100%) | Pneumococcal bacterial pneumonia n (%) |
Atypical bacterial pneumonia n (%) |
Magnitude (95% CI) |
p value |
|
Age ≤2 years (n = 14) >2 years (n = 45) |
9 (29.03) 22 (70.97) |
5 (17.86) 23 (82.14) |
OR: 1.88 (0.54 to 6.50) |
0.4832 |
|
Age ≤5 years (n = 41) >5 years (n = 18) |
29 (93.55) 2 (6.45) |
12 (42.86) 16 (57.14) |
OR: 19.33 (3.84 to 97.36) |
<0.001 |
|
Age in months, mean (SD) |
32.68 (21.14) |
74.25 (42.21) |
DM: -41.57 (-59.45 to 23.69) |
<0.001 |
|
Tachypnoea Yes (n = 28) No (n = 26) |
10 (35.71) 18 (64.29) |
18 (69.33) 8 (30.77) |
OR: 0.25 (0.08 to 0.77) |
0.0285 |
|
Hypoxaemia (Sat O2 ≤92%) Yes (n = 4) No (n = 51) |
1 (3.23) 3 30 (96.77) |
3 (12.50) 21 (87.50) |
OR: 0.23 (0.02 to 2.40) |
0.4295 |
|
Moderate chest retractions (subcostal/intercostal) Yes (n = 5) No (n = 54) |
5 (16.13) 26 (83.87) |
0 (0) 28 (100) |
RP: 2.08 (1.57 to 2.74) |
0.0796 |
|
Conjunctivitis Yes (n = 0) No (n = 59) |
0 (0) 31 (100) |
0 (0) 28 (100) |
|
|
|
Nasal congestion Yes (n = 34) No (n = 25) |
22 (70.97) 9 (29.03) |
12 (42.86) 16 (57.14) |
OR: 3.36 (1.11 to 9.57) |
0.0511 |
|
Admission Yes (n = 6) No (n = 53) |
4 (12.90) 27 (87.10) |
2 (7.14) 26 (92.86) |
OR: 1.93 (0.32 to 11.43) |
0.7644 |
|
CRP (mg/dl) media (SD)
|
4.60 (10.18) |
1.54 (1.70) |
DM: 3.06 (-0.72 to 6.84) |
0.7544 |

| Table 5. Summary of main results of the univariate analysis comparing viral pneumonia and atypical pneumonia. Quantitative variables expressed as mean ± standard deviation if they followed a normal distribution (white blood cell count), and otherwise as median and interquartile range (neutrophils and CRP) | ||||
|---|---|---|---|---|
| Total 92 (100%) | Viral pneumonia n (%) |
Atypical bacterial pneumonia n (%) |
Magnitude (95% CI) |
p value |
|
Age ≤2 years (n = 16) >2 years(n = 32) |
11 (55.00) 9 (45.00) |
5 (17.86) 23 (82.14) |
OR: 5.62 (1.52 to 20.80) |
0.0173 |
|
Age ≤5 years (n = 29) >5 years (n = 19) |
17 (85.00) 3 (15.00) |
12 (42.86) 16 (57.14) |
OR: 7.56 (1.79 to 31.81) |
0.0082 |
|
Age in months, mean (SD) |
36.15 (44.51) |
74.25 (42.21) |
DM: 38.10 (12.31 to 63.88) |
<0.001 |
|
Tachypnoea Yes (n = 24) No (n = 22) |
6 (35.29) 14(64.71) |
18 (69.33) 8 (30.77) |
OR: 0.19 (0.05 to 0.68) |
0.0605 |
|
Hypoxaemia (Sat O2 ≤92%) Yes (n = 8) No (n = 35) |
5 (26.32) 14 (73.68) |
3 (12.50) 21 (87.50) |
OR: 2.50 (0.51 to 12.17) |
0.4463 |
|
Moderate chest retractions (subcostal and intercostal) Yes (n = 6) No (n = 42) |
6 (30.00) 14 (70.00) |
0 (0) 28 (100) |
PR: 3.00 (1.95 to 4.60) |
0.0080 |
|
Conjunctivitis Yes (n = 4) No (n = 44) |
4 (20.00) 16 (80.00) |
0 (0) 28 (100) |
PR: 2.75 (1.86 to 4.06) |
0.0211 |
|
Nasal congestion Yes (n = 30) No (n = 18) |
18 (90.00) 2 (10.00) |
12 (42.86) 16 (57.14) |
OR: 12.00 (2.32 to 61.95) |
0.0022 |
|
Admission Yes (n = 12) No (n = 36) |
10 (50.00) 10 (50.00) |
10 (50.00) 10 (50.00) |
OR: 13.00 (2.41 to 70.05) |
0.0023 |
|
CRP (mg/dl), mean (SD) |
7.63 (9.57) |
1.79 (1.71) |
DM: -5.83 (-10.49 to -1.19) |
0.0111 |

Lastly, the concordance between the initial diagnosis of suspected atypical pneumonia and the final diagnosis of atypical pneumonia confirmed by positive serological test results corresponded to a Fleiss kappa coefficient of 0.26, which represents a weak correlation (Table 6).
| Table 6. Absolute frequency distribution of M. pneumoniae serologic tests results according to the suspected pneumonia diagnosis | |||
|---|---|---|---|
| Positive serology | Negative serology | Total | |
| Atypical pneumonia | 6 | 4 | 10 |
| Negative serology | 22 | 44 | 66 |
| Total | 28 | 48 | 76 |

Thus, the diagnosis of suspected atypical pneumonia was only confirmed in 6 out of 28 cases (21.4%). However, the diagnosis of non-atypical pneumonia turned out to be correct in 91.6% of cases (44 out of 48). The PPV of suspicion based on clinical/radiographic features was 60% (95% CI: 29.6 to 90.3%) and the NPV was 66.6% (95% CI: 55.3 to 78.1%). The sensitivity of the clinical diagnosis of atypical pneumonia was 21.4% (95% CI: 6.2 to 36.6%), the specificity 91.6% (95% CI: 83.85% a 99.4%), the false positive rate (FPR) 8.3%, the false negative rate (FNR) 78.5%, the positive likelihood ratio (LR+) 2.5 (95% CI: 0.8 to 8.3) and the negative likelihood ratio (LR−) 0.8 (95% CI: 0.6 to 1.1). These likelihood ratios (according to the Evidence-Based Medicine Working Group) are in ranges that correspond to small (but sometimes important) changes in probability.
DISCUSSION
Our study included a total of 92 patient, of who 28 had pneumonia caused by M. pneumoniae, in most cases diagnosed at the PC level and with a favourable outcome with empiric antibiotherapy.
In this case series, atypical pneumonia was diagnosed in 30% of the patients and mixed pneumonia in 4% in whom M. pneumoniae was associated with another pathogen, usually a virus. Eighty percent of positive serologic tests for M. pneumoniae occurred in patients older than 5 years, however, in the remaining age groups, the proportion of positive tests was as high as 30%, a figure that ought to be taken into account when it comes to suspecting atypical aetiological agents in other ages.12-15 These findings were consistent with those of other studies, which have reported a frequency of atypical agents of 30-40%.16-18 Still, there is no consensus as to the role of coinfection with M. pneumoniae and a viral agent in terms of prognosis and outcomes, and it is not possible to determine whether these cases of infection by both agents are cases of concurrent coinfection or secondary superinfection.19,20
Thus, we were able to establish the approximate incidence of infection by atypical pathogens in our catchment population. We found that a high proportion of these infections occurred in children under 5 years, with a mean age below the one traditionally described. The high proportion may be explained by enrolment in educational facilities and therefore sensitization that predisposes to more severe infection with additional exposure to the microorganism at earlier ages. But it could also be attributed to a limitation of the study, as the diagnosis was based on IgM antibody tests and IgM levels do not increase during reinfection, when there is a rapid IgG and IgA antibody response that was not assessed in the sample, so that some cases may have been missed in school-age children and adolescents. Furthermore, a hospital-based study by Wood et al,17 among others, found a 56% prevalence of colonization by M. pneumoniae in healthy children using PCR tests in nasopharyngeal samples and serologic tests (Ig M and Ig G).
Notwithstanding, the proportion of atypical pneumonia in our study was relevant and consistent with the recent literature,2,5,26 which suggests that this type of pneumonia is underdiagnosed in the paediatric population and that its actual incidence remains unknown.
Age was one of the main variables under study and, consistent with the previous literature, atypical pneumonia was more frequent in children aged more than 60 months. Until recently, age was considered the chief predictor of the type of pneumonia.21-24 In agreement with the majority of the published series,23,24,27,28 the mean age was significantly higher in cases of atypical pneumonia compared to all other types.
Chief among the most frequent manifestations in cases of pneumonia caused by M. pneumoniae were fever, cough, nasal congestion, general malaise, gastrointestinal symptoms and tachypnoea, with a significant difference in the latter compared to non-atypical pneumonia, especially cases of suspected pneumococcal aetiology. Few studies have explored this aspect,29 and this was an interesting finding given that this form of pneumonia tends to have a more insidious onset, so that diagnosis may be delayed. Some of these studies only found patients with tachypnoea when pneumonia involving M. pneumoniae was associated with viral infection.29 Similarly, and contrary to the most insidious course of this type of pneumonia, Søndergaard et al29 found that cough was found in 100% of cases of atypical pneumonia with tachypnoea.
On the other hand, rib pain was three times more frequent in atypical pneumonia cases compared to the rest, a finding that may have been influenced by the higher age of the patients, who had the ability to verbalise subjective symptoms such as pain
Among the cases with an alveolar pattern, the proportions of atypical and non-atypical pneumonia were similar, contrary to previous descriptions10 ,and consistent with the most recent studies, in which no specific pattern appears to be exclusively associated with a particular aetiology.5,6,22,30. Lobar consolidation can no longer be considered pathognomonic of typical bacterial pneumonia, since it is found in a large proportion of cases of atypical pneumonia that increases with viral coinfection.23,29,30
Another finding worth highlighting is that the relative frequency of pleural effusion was very similar in cases of pneumonia caused by M. pneumoniae and cases of a non-atypical aetiology, which was similar to the results of recent studies that have found proportions of up to 18% in cases associated with viral infection.29 Generally, these are small effusions with little clinical impact.
On the other hand, we found that laboratory markers were nonspecific in regard to the bacterial aetiology and only provided information that could complement the diagnosis, as demonstrated by other authors.31,32 In addition, the efficacy of serologic testing as the sole diagnostic method is particularly controversial in the case of M. pneumoniae, and it has been demonstrated that its use in combination with polymerase chain reaction (PCR) for detection of bacterial DNA is more efficacious. Further advance in this field need to occur before it is possible to obtain quick and reliable results that can guide decision-making at the PC level.
Most patients in our series received amoxicillin via the oral route, used correctly as monotherapy in 80% of cases of non-atypical pneumonia, but it was also prescribed in half the cases of atypical pneumonia, with good outcomes. Only one fourth of atypical cases were treated with macrolides based on the suspected diagnosis, and we found no differences in the outcomes compared to treatment with a beta-lactam antibiotic, a finding that was consistent with the results of some systematic reviews.18,33 We ought to underscore the resolution of symptoms with the use of amoxicillin, without needing to resort to a macrolide, despite the detection of M. pneumoniae. The benefits of antibiotherapy in children with infection by M. pneumoniae have not been studied adequately.33 Several systematic reviews18,33,34 have yielded similar results, according to which the benefits of using macrolides to treat pneumonia caused by M. pneumoniae are unclear in children. In consequence, some authors consider that antibiotherapy is unnecessary in these atypical respiratory infections, as they tend to be self-limiting, although antibiotic treatment would reduce the duration of symptoms and the rate of transmission. This evinces the pressing need to improve the initial aetiological diagnosis.
One of the main limitations of the study concerns the statistical power derived from the sample size (n = 92), which may have led to results that were not statistically significant but could have been, indicating a changing trend, if more cases had been included. We applied a classification of the type of pneumonia that took into account the limitations or unavailability of the aetiological diagnosis methods in the PC setting. Therefore, the assumptions made in the study call for caution in the interpretation of its results.
Due to limitations in the aetiological diagnosis methods available at the PC level, we considered valid positive results for IgM against M. pneumoniae obtained through a qualitative method (ELISA) in the first week from onset. It is known that there are no tests sensitive enough to allow a rapid and reliable diagnosis of infection by M. pneumoniae. The management guideline of the IDSA recommends performance of serologic testing (in the acute phase and the convalescent phase) or PCR testing (which is quicker and highly specific) in nasopharyngeal secretion samples. However, the increased cost and resources this would require in our PC catchment area precluded the application of these techniques.
Lastly, this study contributes data demonstrating that there are few epidemiological, clinical or radiographic differential features of the different types of pneumonia considered at present that can be used to guide the aetiological diagnosis, especially if atypical pathogens are involved.
ABBREVIATIONS
CAP: community-acquired pneumonia · CI: confidence interval · CRP: C-reactive protein · NPA: nasopharyngeal aspirate · LR−-: negative likelihood ratio · LR+: positive likelihood ratio · FNR: false negative rate · FPR: false positive rate · NPV: negative predictive value · PPV: positive predictive value · RR: respiratory rate · RSV: respiratory syncytial virus.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article.
This research project was partially funded through the 2018 José María Mengual Mur grant of the Fundación para el Progreso de la Pediatría.
REFERENCES
- Andrés Martín A, Moreno-Pérez D, Alfayate Miguélez S, Couceiro Gianzo JA, García García ML, Korta Murua J, et al. Etiología y diagnóstico de la neumonía adquirida en la comunidad y sus formas complicadas. An Pediatr (Barc). 2012;76:162.e1-162.e18.
- Honkinen M, Lahti E, Osterback R, Ruuskanen O,Waris M. Viruses and bacteria in sputum samples of children with community-acquired pneumonia. Clin Microbiol Infect. 2012;18:300-7.
- Infants and Children: Acute Management of Community Acquired Pneumonia. In: NSW Health. Guideline. Agency for Clinical Innovation; 2018 [online] [accessed 15/09/2022]. Available at www1.health.nsw.gov.au/pds/ActivePDSDocuments/GL2018_007.pdf
- Aguilera-Alonso D, Illán-Ramos M, Daoud Z, Guinea V, Culebras E, Ramos JT. Análisis del impacto de los test de diagnóstico virológico en el consumo de antibióticos en pacientes pediátricos ingresados por neumonía adquirida en la comunidad. Enferm Infecc Microbiol Clin. 2020;38:230-3.
- Nelson KA, Morrow C, Wingerter SL, Bachur RG, Neuman MI. Impact of chest radiography on antibiotic treatment for children with suspected pneumonia. Pediatr Emerg Care. 2016;32:514-9.
- Neuman MI, Graham D, Bachur R. Variation in the use of chest radiography for pneumonia in pediatric emergency departments. Pediatr Emerg Care. 2011;27:606-10.
- Clark JE, Hammal D, Hamptom F, Spencer D, Parker l. Epidemiology of community-acquired pneumonia in children seen in hospital. Epidemiol Infect. 2007;135:262-9.
- Heiskanen-Kosma T, Korppi M, Jokinen C, Kurki S, Heiskanen l, Juvonen H, et al. Etiology of childhood pneumonia: Serologic results of a prospective, population-based study. Pediatr Infect Dis J. 1998;17:986-91.
- The Management of acute respiratory infections in children: practical guidelines for outpatient care. In: World Health Organization; 1995 [online] [accessed 15/09/2022]. Available at https://apps.who.int/iris/handle/10665/41803
- Giménez Sánchez F, Sánchez Marenco A, Battles Garrido JM, López Soler JA, Sánchez-Solís Querol M. Características clínico-epidemiológicas de la neumonía adquirida en la comunidad en niños menores de 6 años. An Pediatr (Barc). 2007;66:578-84.
- Rudan I, O’Brien KL, Nair H, Liu l, Theodoratou E, Qazi S, et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. 2013;3:010401.
- Korppi M, Heiskanen-Kosma T, Kleemola M. Incidence of community-acquired pneumonia in children caused by pneumoniae: serological results of a prospective, population-based study in primary health care. Respirology. 2004;9:109-14.
- Layani-Milon MP, Gras I, Valette M, Luciani J, Stagnara J, Aymard M, et al. Incidence of upper respiratory tract pneumoniae infections among outpatients in Rhone-Alpes, France, during five successive winter periods. J Clin Microbiol. 1996;34:447-9.
- Rasmussen JN, Voldstedlund M, Andersen RL, Ellermann-Eriksen S, Jensen TG, Johansen HK, et al. Increased incidence of pneumoniae infections detected by laboratory-based surveillance in Denmark in 2010. Euro Surveill. 2010;15:19708.
- Jacobs E. Pneumoniae disease manifestations and epidemiology. In: Razin S, Herrman R, editores. Molecular biology and pathogenicity of mycoplasmas. Kluwer New York: Academic/Plenum Publishers; 2002. p. 519-30.
- Baer G, Engelcke G, Abele-Horn M, Schaad UB, Heininger U. Role of Chl. pneumoniae and pneumoniae as causative agents of community-acquired pneumonia in hospitalised children and adolescents. Eur J Clin Microbiol Infect Dis. 2003;22:742-5.
- Wood PR, Hill VL, Burks ML, Peters JI, Singh H, Kannan TR, et al. Mycoplasma pneumoniae in children with acute and refractory asthma. Ann Allergy Asthma Immunol. 2013;110:328-34.
- Mulholland S, Gavranich JB, Gillies MB, Chang AB. Antibiotics for community acquired lower respiratory tract infections secondary to pneumoniae in children. The Cochrane Database of Systematic Reviews 2012;9:CD004875.
- Cimolai N, Wensley D, Seear M. Mycoplasma pneumoniae as a cofactor in severe respiratory infections. Clin Infect Dis. 1995;21:1182-5.
- Cemeli Cano M, Laliena Aznar S, Beltrán García S, Sáez de Adana Pérez ME y García Vera C. Neumonía muy atípica en paciente de dos años. Rev Pediatr Aten Primaria. 2019:21:61-4.
- Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, Thomson A. British Thoracic Society guidelines for the management of community acquired pneumonia in children. Thorax. 2011;66:1-23
- Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:25-76.
- Úbeda Sansano MI, Murcia García J, Asensi Monzó MT y Grupo de Vías Respiratorias. Neumonía adquirida en la comunidad. El pediatra de Atención Primaria y la Neumonía. Protocolo del GVR (publication P-GVR-8) [online] [accessed 15/09/2022]. Available at www.aepap.org/sites/default/files/documento/archivos-adjuntos/protocolo-neumonia-2017.pdf
- Byington CL, Bradley JS. Pediatric community-acquired pneumonia. In: Feigin and Cherry’s Textbook of Pediatric Infectious Diseases, 7th ed, Cherry JD, Harrison GJ, Kaplan SL, Steinbach W, Hotez P (Eds), Elsevier Saunders, Philadelphia 2014; 283.
- Clark JE, Hammal D, Spencer D, Hampton F. Children with pneumonia: how do they present and how are they managed? Arch Dis Child. 2007;92:394-8.
- Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835-45.
- Garcés-Sánchez MD, Díez-Domingo J, Ballester Sanz A, Peidro Boronat C, García López M, Antón Crespo V, et al. Epidemiología de la neumonía adquirida en la comunidad en menores de 5 años en la Comunidad Valenciana. An Pediatr (Barc). 2005;63:125-30.
- Jiang W, Wu M, Zhou J, Wang Y, Hao C, Ji W, et al. Etiologic spectrum and occurrence of coinfections in children hospitalized with community-acquired pneumonia. BMC Infectious Diseases. 2017;17:787-95.
- Søndergaard MJ, Friis MB, Hansen DS, Jørgensen IM. Clinical manifestations in infants and children with pneumoniae infection. PLoS One 2018 Apr 26;13(4):e0195288.
- Finnegan OC, Fowles SJ, White RJ. Radiographic appearances of pneumoniae. Thorax. 1981;36:469-72.
- Summah H, Qu JM. Biomarkers: A definite plus in pneumonia. Mediators Inflamm. 2009;6753-62.
- Peltola V, Mertsola J, Ruuskanen O. Comparison of total white blood cell count and serum C reactive protein levels in confirmed bacterial and viral infections. J Pediatr. 2006;149:721-4.
- Biondi E, McCulloh R, Alverson B, Klein A, Dixon A, Ralston S. Treatment of pneumoniae: A systematic review. Pediatrics. 2014;133:1081-90.
- Gardiner SJ, Gavranich JB, Chang AB. Antibiotics for community-acquired lower respiratory tract infections secondary to pneumoniae in children. Cochrane Database Syst Rev. 2015; 1:CD004875.





