Vol. 24 - Num. 94
Original Papers
Vaccination coverage against pertussis and influenza in pregnant women managed in an urban primary care centre
Raquel González Olallaa, Juana Paredes Garridob
aPediatra. CS Entrevías. Madrid. España.
bEnfermera. CS Entrevías. Madrid. España.
Correspondence: R González. E-mail: rgolalla@gmail.com
Reference of this article: González Olalla R, Paredes Garrido J. Vaccination coverage against pertussis and influenza in pregnant women managed in an urban primary care centre. Rev Pediatr Aten Primaria. 2022;24:e201-e206.
Published in Internet: 20-06-2022 - Visits: 10360
Abstract
Introduction: pertussis is a highly contagious infectious disease. In infants, the severity of pertussis and its complications requires hospitalization in most cases and the associated mortality is high. Vaccination against specific diseases is particularly indicated in pregnancy, for instance influenza (inactivated vaccine) and pertussis.
Material and methods: we conducted an observational descriptive study. The study sample consisted of mother-child dyads selected through the identification of newborns assigned to the caseload of the Entrevías Primary Care Centre between January 2019 and March 2020. We collected data on the age, vaccination schedule of the pregnant woman, and episodes of pertussis documented in the health records of the infant in the first 2 months post birth.
Results: the vaccination coverage in pregnant women was 83.5% for the diphtheria, tetanus and acellular pertussis (Tdap) vaccine and 39.5% for the influenza vaccine. There were 3 cases of pertussis in infants aged less than 3 months in the group of unvaccinated mothers, one of who died at 1 month post birth, and no cases in the group of vaccinated mothers.
Conclusions: the influenza vaccination coverage in pregnant women is below the recommended target, which evinces the need of measures to improve vaccination coverage in pregnant women. In the group of infants of mothers not vaccinated with the Tdap, there were 3 cases of pertussis (7%), and there were no cases in the group of infants of vaccinated mothers.
Keywords
● Influenza vaccine ● Pertussis vaccine ● Pregnancy ● Vaccination coverageINTRODUCTION
Prenatal care visits are a great opportunity for catch up vaccination in pregnant women and to inform them that the benefits of vaccination outweigh its risks.1 Some vaccines are particularly indicated during pregnancy, such as the inactivated influenza vaccine and the pertussis vaccine.2,3
The morbidity and mortality that influenza may cause during pregnancy are similar to those described in other risk groups.4 Infection by influenza in the first trimester of pregnancy is associated with a higher frequency of cardiac malformations, cleft palate and neural tube defects in the baby, and in the second and third trimesters to a higher frequency of miscarriage and preterm birth.5 Vaccination of pregnant women has a triple effect: it protects the mother, protects the newborn and protects infants in the first months of life, with evidence of a 20% decrease in the incidence of severe pneumonia in the offspring of vaccinated women.6
During the flu vaccine campaign, administration of one dose of inactivated influenza vaccine is recommended in pregnant women in any trimester of the pregnancy.7
The goal of vaccination against pertussis is to protect the infant from this disease in the first 3 months of life. Administration of the diphtheria, tetanus and acellular pertussis (Tdap) vaccine is recommended in each pregnancy.
Pertussis is a highly contagious respiratory infection caused by Bordetella pertussis, a gram-negative coccobacillus that, while affecting every age group, is particularly severe in the first months of life8. The associated mortality in infants is high.9,10
The resurgence of pertussis, with important outbreaks emerging in several countries, including those in California and England associated with a substantial increase in the mortality due to this disease in infants under 3 months, has prompted the contemplation of new strategies for prevention and control.9,11 Contact studies12,13 show that parents and other household members are the main sources of transmission to infants that have not yet started vaccination. Vaccination of pregnant women in the third trimester is currently recommended as the most effective and efficient immunization strategy to protect infants before the start of the childhood vaccination series.9 This strategy has proven safe and effective in the prevention of other vaccine-preventable infectious diseases, such as tetanus or influenza.14 The transfer through the placenta of antibodies against pertussis to the foetus is meant to protect the infant until vaccination starts 2 months post birth. At the same time, it confers protection to the mother, which also indirectly protects the newborn. Thus, a single intervention can protect both mother and child without increasing the risk of adverse events.15,16
In the Community of Madrid, since March 28, 2016, pregnant women are vaccinated against pertussis between 28 and 36 weeks of gestation.
The death in January 2020 of a patient aged 1 month assigned to the Entrevías PCC (Madrid), born to a mother that was not vaccinated during pregnancy, motivated us to analyse vaccination status in pregnant women in the caseload of the centre.
MATERIAL AND METHODS
Design: observational descriptive study. Setting: Entrevías primary care centre (PCC) in Madrid, Spain. Study universe: the sampling unit was the mother-child dyad, and dyads were included through the identification of newborns allocated to the caseload of the Entrevías PCC between January 2019 and March 2020. Inclusion criteria: patients in the caseload of the PCC born between January 2019 and March 2020.
Sociodemographic variables: sex; current age; vaccination history of the mother; correct vaccination of mother against pertussis in third trimester (yes/no); correct vaccination of mother against influenza during pregnancy (yes/no); episodes of pertussis documented in the health records in the first 2 months post birth.
Data collection: the data were collected through the review of the health records of patients meeting the inclusion criteria in the health information system of the primary care system of Madrid. Once the data relevant to the study were collected, personal information was dissociated and a file created that included only anonymised data. The researchers committed to safeguarding the confidentiality of the data.
Analysis strategy: the data were processed and analysed with the software SPSS version 21. We described quantitative variables using the mean and standard deviation (SD) if the data followed a normal distribution, and using the median and interquartile range otherwise. We described qualitative variables through the absolute and relative frequency distributions of their categories.
To pursue the goal of the study, we calculated the relevant percentages. These are presented as single time point estimates with the corresponding 95% confidence intervals (CIs).
RESULTS
A total of 261 newborns were assigned to the Entrevías PCC between January 2019 and March 2020 (138 male and 123 female). The median maternal age was 30.06 years (SD, 6.7; range, 15 -47); the vaccination histories of 73 mothers (28%) were included in the electronic health records system of the primary care system of Madrid; 103 mothers (39.5%) received the influenza vaccine during the pregnancy (Figure 1) and 218 (83.5%) the Tdap vaccine (Figure 2).
| Figure 1. Influenza vaccination coverage in pregnant women at the Entrevías primary care centre (Madrid) between January 2019 and March 2020 |
|---|
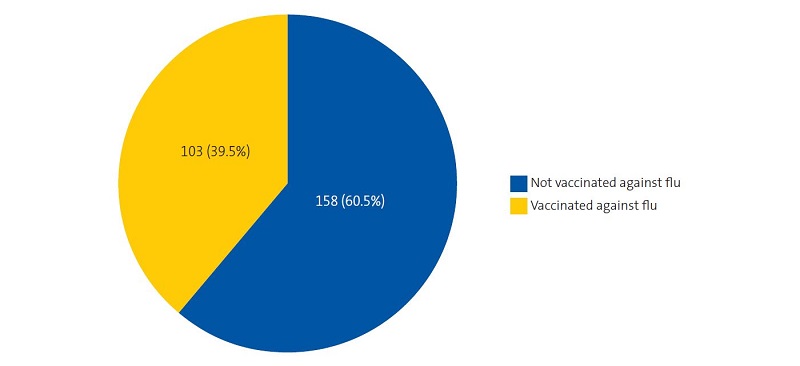 |

| Figure 2. Coverage of vaccination with the Tdap vaccine in pregnant women at the Entrevías primary care centre (Madrid) between January 2019 and March 2020 |
|---|
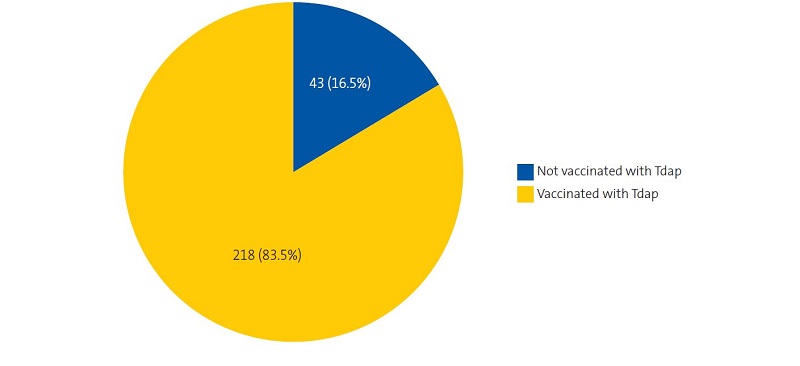 |

By age group, the lowest proportion of vaccination was found in pregnant women aged less than 25 years.
Pertussis was diagnosed within 2 months of birth in 3 infants, with microbiological confirmation in 2, and 1 of these infants died at 1 month post birth. In all 3 cases, the mothers had not been vaccinated with the Tdap vaccine. None of the offspring of vaccinated mothers had pertussis, compared 7% of the offspring of unvaccinated women. This association was statistically significant (p <0.001).
DISCUSSION
This study provides information on the adherence to the influenza and Tdap vaccination programme in pregnant women between January 2019 and March 2020. We found that 72% of pregnant women did not have their vaccination history documented in their health records (HRs). A possible explanation is that in the Community of Madrid, the transition from paper-based to electronic health records took place in 2004, and at the time vaccination histories were only transcribed from year 2000, which means that in women aged more than 19 years, the vaccination history may only be documented in paper or may be difficult to access if the patient comes from other regions in Spain or other countries.
We found a high vaccination coverage for the Tdap vaccine (83.5%), similar to the overall coverage in Spain in 2019 (83.6%) and lower than the coverage in Madrid (92.7%).
The influenza vaccination coverage in pregnant women in our PCC, whose data are presented in Table 1, was 39.5%, below the overall coverage for Spain, which was 50%, and for the Community of Madrid, which was 55.8%.
| Table 1. Vaccination with Tdap and maternal age | |||||
|---|---|---|---|---|---|
| Tdap vaccine | Total | ||||
| No | Yes | ||||
| Age (years) | 15 to 25 | % in age group | 33.8% | 66.2% | 100.0% |
| 26 to 35 | % in age group | 12.3% | 87.7% | 100.0% | |
| 36 or greater | % in age group | 6.6% | 93.4% | 100.0% | |
| Total | % in age group | 16.4% | 83.5% | 100.0% | |

The incidence of pertussis in the period under study was 7% in the infants born to unvaccinated mothers.
Contacts with the health care system during pregnancy should be used as opportunities to assess vaccination status in women and vaccinate if indicated: against pertussis, between weeks 28 and 36 of gestation, and against influenza, during the seasonal vaccination campaign in any trimester of the pregnancy.
CONCLUSION
The influenza vaccination coverage in pregnant women is below recommended levels, which evinces the need of measures to improve vaccination coverage in this group. During the study period, in the group of children born to mothers that did not receive the Tdap vaccine there were 3 cases of pertussis (7%), one of which resulted in death, compared to none in the group of vaccinated mothers.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article.
ABREVIATURAS
CI: confidence interval · Tdap: reduced-antigen content tetanus, diphtheria, and acellular pertussis vaccine · HR: health record · PCC: primary care centre · SD: standard deviation.
REFERENCES
- Vacunación en grupos de riesgo de todas las edades y en determinadas situaciones. Grupo de trabajo vacunación en población adulta y grupos de riesgo de la Ponencia de Programa y Registro de Vacunaciones. Comisión de Salud Pública del Consejo Interterritorial del Sistema Nacional de Salud. In: Ministerio de Sanidad, Consumo y Bienestar Social, July 2018 [online] [accessed 30/05/2022] Available at www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/programasDeVacunacion/riesgo/docs/VacGruposRiesgo_todas_las_edades.pdf
- Housey M, Zhang F, Miller C, Lyon-Callo l, McFadden J, Garcia E, et al. Vaccination with tetanus, diphtheria, and acellular pertussis vaccine of pregnant women enrolled in Medicaid-Michigan, 2011-2013. MMWR Morb Mortal Wkly Rep. 2014;63:839-42.
- Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Klein NP, Cheetham TC, Naleway AL, et al. Maternal Tdap vaccination: Coverage and acute safety outcomes in the vaccine safety datalink, 2007-2013. Vaccine. 2016;34:968-73.
- Departament de Salut. Generalitat de Catalunya. Malalties de declaració obligatòria: numèrica i individualitzada. Butlletí Epidemiol Catalunya (BEC). 2014;166:177.
- Vilajeliu A, Urbiztondo l, Martínez M, Batalla J, Cabezas C. Vacunació de les dones embarassades contra la tos ferina a Catalunya. Agència de Salut Pública de Catalunya. Barcelona; 2014.
- La vacunació contra la tos ferina en dones embarassades s’associa a una reducció important de casos en nadons. February 24, 2015 [online] [accessed 31/05/2022]. Available at https://capramblaferranics.wordpress.com/2015/03/04/la-vacunacio-contra-la-tos-ferina-en-dones-embarassades-sassocia-a-una-reduccio-important-de-casos-en-nadons/
- Pérez XM, Ricart A, Rufach A, Torrabias M, Juvany A, Cabral M, et al. Avaluació del programa de vacunació de la tos ferina en embarassades a la comarca d’Osona durant l’any 2014. Poster presented at the XXI Annual Meeting of the Societat Catalana de Pediatria. May 8-9, 2015, Manresa [online] [accessed 20/05/2022]. Available at http://postersdigitals.academia.cat/posterDetall.php?idDiapo=2155
- Grande Tejada AM. Actualización en tosferina. Rev Pediatr Aten Primaria. 2016;18:41-6.
- Fernández-Cano MI, Espada-Trespalacios X, Reyes-Lacalle A, Manresa Domínguez JM, Armadans-Gil l, Campins-Martí M, et al. Cobertura vacunal frente a tos ferina en gestantes de Cataluña en el primer año de implantación del programa de inmunización. Enferm Infecc Microbiol Clin. 2017;35:550-5.
- Campins M, Moreno-Pérez D, Gil-de Miguel A, González-Romo F, Moraga-Llop FA, Arístegui-Fernández J, et al. Tosferina en España. Epidemiología actual, estrategias de prevención y control. Recomendaciones del Grupo de trabajo contra la tosferina. Enferm Infecc Microbiol Clin. 2013;31:240-53.
- Fernández-Cano MI, Armadans-Gil l, Alvarez-Bartolomé M, Rodrigo-Pendás JA, Campins-Martí M. Hospitalización por tos ferina en España (1997-2011). Enferm Infecc Microbiol Clin. 2014;32:638-42.
- Winter K, Harriman H, Zipprich J, Schechter R, Talarico J, Watt J, et al. California pertussis epidemic, 2010. J Pediatr. 2012;161;1091-6.
- Wiley KE, Zuo Y, Macartney KK, McIntyre PB. Sources of pertussis infection in young infants: A review of key evidence informing targeting of the cocoon strategy. Vaccine. 2013;31:618-25.
- Uriona Tuma SM, Martínez Gómez X, Campins Martí M, Codina Grau G, Ferrer Marcelles A, Rodrigo Pendás JA, et al. Seguimiento de contactos de casos de tos ferina pediátrica en un hospital terciario de Barcelona, España. Med Clin (Barc). 2013;141:376-81.
- Beigi RH, Fortner KB, Munoz FM, Roberts J, Gordon JL, Han HH, et al. Maternal immunization: Opportunities for scientific advancement. Clin Infect Dis. 2014;59:S408-14.
- Munoz FM, Bond NH, Maccato M, Pinell P, Hammill H, Swamy GK, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: A randomized clinical trial. JAMA. 2014;311:1760-9.





