Vol. 24 - Num. 93
Original Papers
Evolution of vaccination against serogroup B meningococcus: what has changed in 5 years?
María Vázquez Sáncheza, Cristina Genzor Ríosb, David Molina Herranza, M.ª Violeta Fariña Jaraa, Mónica López Camposc, Carmen Puig Garcíac, Carmen Viñas Viamonted, Enrique Llamas Agúndeze
aMIR-Pediatría. Hospital Universitario Miguel Servet. Zaragoza. España.
bEIR Pediatría. Hospital Universitario Miguel Servet. Zaragoza. España.
cPediatra. CS Actur Norte. Zaragoza. España.
dEnfermera de Pediatría. CS Actur Norte. Zaragoza. España.
eInformático. Sector I. Zaragoza. España.
Correspondence: M Vázquez. E-mail: mvazquezsanc@gmail.com
Reference of this article: Vázquez Sánchez M, Genzor Ríos C, Molina Herranz D, Fariña Jara MV, López Campos M, Puig García C, et al. Evolution of vaccination against serogroup B meningococcus: what has changed in 5 years? Rev Pediatr Aten Primaria. 2022;24:39-46.
Published in Internet: 25-02-2022 - Visits: 11105
Abstract
Introduction: infection by serogroup B meningococcus can cause invasive meningococcal disease, with development of sequelae in 20-30% of cases and a mortality of up to 10%.
Material and methods: observational, descriptive and retrospective study of vaccination against serogroup B meningococcus in the paediatric population of health sector I of Zaragoza between October 2015 and December 2019. We analysed the age at primary vaccination, age group at time of first dose (≤3 months, 4-11 months, 12-23 months, 2-9 years, 10-16 years), sex, primary care centre (PCC) and number of received doses.
Results: 11 776 patients were vaccinated, of who 51.6% were male. The mean age at initiation of vaccination was 5.0 ± 4.4 years, and they received a mean of 2.2 ± 0.6 doses. The distribution of vaccinated patients by PCC was heterogeneous, with a difference of 17.8% between the centre with the most vaccinated patients and the centre with the least. Of all patients, 0.7% received the first dose in 2015, 23.8% in 2016, 38% in 2017, 26.7% in 2018 and 10.8% in 2019. Twelve percent were aged 3 months or less when they received the first dose, 11.5% 4-11 months, 6.7% 12-23 months, 50.4% 2-9 years and 19.5% 10-16 years, with differences based on the date of the first dose (p = 0.000). The highest frequency of overall vaccination corresponded to 2017 (12.2%), although in children under 2 years it was higher in 2018 (42.1%) and in children aged 2-9 years and adolescents it was highest in 2017: 15.8% and 5.4%, respectively. The cumulative frequency of vaccination was 32.5% in the overall sample and 133.5% in the group aged less than 2 years.
Conclusions: although we found promising cumulative vaccination rates, there were numerous differences in vaccination between age groups and PCCs, which is why publicly funded routine vaccination against meningococcus B is worth contemplating.
Keywords
● Meningococcal disease ● Serogroup B meningococcus ● VaccinationINTRODUCTION
Meningococcal disease is caused by the bacterium Neisseria meningitidis, a gram-negative aerobic diplococcus for which humans are the reservoir. Twelve serogroups of this pathogen have been described, the most relevant of which are: A, B, C, W, X, Y. Infection by this pathogen can cause invasive meningococcal disease (IMD) in the form of meningitis and/or sepsis, with sequelae in 20-30% of cases and a mortality of up to 10%.1-3
In Spain and Europe, the estimated incidence of IMD is of 0.3/100 000 inhabitants and 0.2 to 14/100 000 inhabitants, respectively, with a predominance of serogroup B. This may be partly due to routine vaccination against serogroup C. The infection rate varies depending on age, and this is the leading cause of bacterial meningitis in children, chiefly before age 2 or 3 years.2,4
The first vaccine against serogroup B (4CmenB, Bexsero) was authorised on January 14, 2013 after a positive review by the European Medicines Agency. Subsequently, regulatory agencies at the national level had the authority concerning its potential inclusion in the immunization schedule. The United Kingdom was one of the first countries to include it, in March 2014, with a 2 + 1 vaccination series.2
In Spain, the 4CmenB vaccine has been officially available since August 13, 2014 in hospital pharmacies and since 2015 in community pharmacies as a vaccine that is not funded by the public health system for the general population, except for Castilla y León and Canary Islands, where it is publicly funded.1,2 At present, 2 vaccines against serogroup B are commercially available in Spain: Bexsero, authorised for use from age 2 months, and Trumemba, authorise from age 10 years.1
Objectives: 1) to assess meningococcal B vaccination coverage from the time it started being distributed in Spain through December 2019, before the start of the SARS-CoV-2 pandemic, through a study conducted in Zaragoza; 2) to assess differences in vaccination between age groups and 3) to study possible differences in vaccination frequency between primary care centres (PCCs).
MATERIAL AND METHODS
We conducted a retrospective, observational and study through the collection of data on paediatric patients (up to age 16 years) vaccinated against serogroup B meningococcus (MenB) in health sector I of Zaragoza from October 2015 to December 2019. We coded the 13 PCCs included in sector I of Zaragoza as letters A through M in succession.
The final sample comprised a total of 11 776 patients. For each patient, we collected information on the following variables: date of birth, age at start of vaccination, age group at time of first dose (≤3 meses, 4-11 months, 12-23 months, 2-9 years, 10-16 years), sex, PCC and number of received doses.
We collected the data and performed the statistical analysis using the software IBM SPSS Statistics 20 for Windows. We expressed the results using measures of central tendency (mean) and of dispersion (standard deviation). We used different statistical tests based on the type of variable under study. We considered results corresponding to a p-value of less than 0.05 statistically significant. The original research project for the study was approved by the Clinical Research Ethics Committee of Aragon.
RESULTS
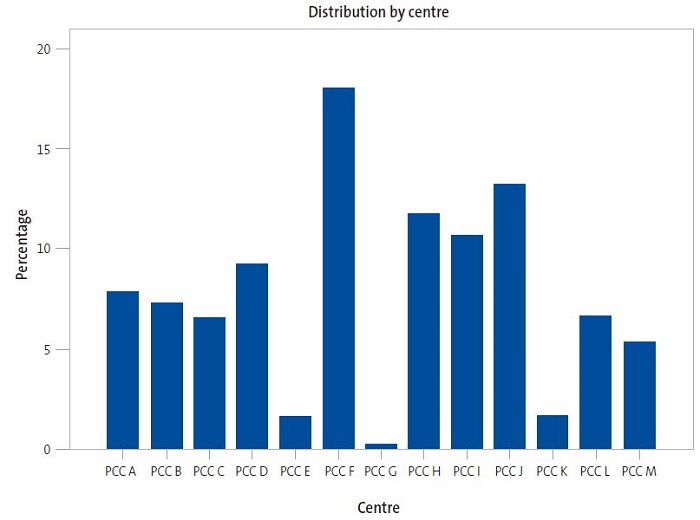
The study included 11 776 patients vaccinated between 2015 and 2019 with a mean age at the time they received the first dose of vaccine of 5.0 ± 4.4 years. Of the total patients, 51.6% were male and 48.5% female. They received a mean of 2.1 ± 0.6 vaccine doses. Eighteen percent of the total vaccinated patients were managed in primary care centre "F" (PCC F), followed by PCC J, which accounted for 13.2% of the vaccinated. Primary care centres G, E and K had the fewest vaccinated patients, corresponding to 0.3%, 1.6% and 1.7% of the total, respectively. Figure 1 shows the distribution of patients by PCC.
| Figure 1. Distribution of total patients vaccinated against MenB by primary care centre in health sector I of Zaragoza |
|---|
 |

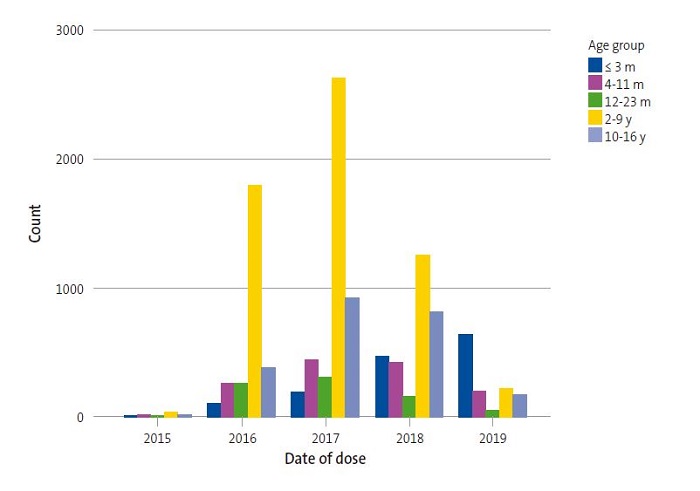
Of all vaccinated patients, 0.7% received the first dose in 2015, 23.8% in 2016, 38% in 2017, 26.7% in 2018 and 10.8% in 2019. As concerns age at the time of administration of the first dose, 12.0% were aged 3 months or less, 11.5% 4 to 11 months, 6.7% 12 to 23 months, 50.4% 2 to 9 years and 19.5% 10 to 16 years. Thus, 30.2% of patients started vaccination before age 2 years. Table 1 and Figure 2 show the differences between age groups in the timing of the first vaccine dose (p = 0.000). We found that as time went by, the relative frequency of vaccination in infants aged less than 1 year has increased, on account of both more frequent vaccination in infants and less frequent vaccination in children aged 2 to 9 years and adolescents, differences that became most marked in 2018 and 2019.
| Table 1. Year of vaccination against serogroup B meningococcus by age group | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age group | Total | |||||||
| <3 months | 4-11 months | 12-23 months | 2-9 months | 10-16 months | ||||
| Date of dose | 2015 | Count | 6 | 13 | 9 | 42 | 12 | 82 |
| % in year | 7.3% | 15.9% | 11.0% | 51.2% | 14.6% | 100.0% | ||
| % in age group | 0.4% | 1.0% | 1.1% | 0.7% | 0.5% | 0.7% | ||
| 2016 | Count | 101 | 266 | 270 | 1792 | 378 | 2807 | |
| % in year | 3.6% | 9.5% | 9.6% | 63.8% | 13.5% | 100.0% | ||
| % in age group | 7.2% | 19.7% | 34.2% | 30.2% | 16.5% | 23.8% | ||
| 2017 | Count | 185 | 443 | 306 | 2625 | 921 | 4480 | |
| % in year | 4.1% | 9.9% | 6.8% | 58.6% | 20.6% | 100.0% | ||
| % in age group | 13.1% | 32.8% | 38.7% | 44.3% | 40.1% | 38.0% | ||
| 2018 | Count | 474 | 439 | 154 | 1258 | 815 | 3140 | |
| % in year | 15.1% | 14.0% | 4.9% | 40.1% | 26.0% | 100.0% | ||
| % in age group | 33.6% | 32.5% | 19.5% | 21.2% | 35.5% | 26.7% | ||
| 2019 | Count | 646 | 188 | 51 | 214 | 168 | 1267 | |
| % in year | 50.9% | 14.8% | 4.1% | 17.0% | 13.2% | 100.0% | ||
| % in age group | 45.8% | 13.9% | 6.5% | 3.6% | 7.3% | 10.7% | ||
| Total | Count | 1412 | 1349 | 790 | 5931 | 2294 | 11776 | |
| % in year | 12.0% | 11.5% | 6.7% | 50.4% | 19.5% | 100.0% | ||
| % in age group | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | ||

We found significant differences in the mean age at administration of the first dose between PCCs (mean age range, 3.5 ± 3.9 to 6.1 ± 4.9 years; p = 0.000). The age at the first dose also varied depending on the year of vaccination (5.6 ± 4.2 in 2017 versus 2.6 ± 4.5 years in 2019; p = 0.000).
| Table 2. Rate of vaccination against serogroup B meningococcus by age group, 2015-2019 | |||||
|---|---|---|---|---|---|
| <24 months | 2-9 years | 10-16 years | Total | ||
| 2015 | N | 3233 | 18 323 | 16 352 | 37 938 |
| Vaccinated (%) | 28 (0,9) | 42 (0,2) | 12 (0,1) | 82 (0,2) | |
| 2016 | N | 3054 | 17 402 | 16 702 | 37 158 |
| Vaccinated (%) | 637 (20,9) | 1792 (10,3) | 378 (2,3) | 2807 (7,6) | |
| 2017 | N | 2830 | 16 589 | 17 183 | 36 602 |
| Vaccinated (%) | 934 (33) | 2625 (15,8) | 921 (5,4) | 4480 (12,2) | |
| 2018 | N | 2536 | 15 578 | 17 518 | 36 632 |
| Vaccinated (%) | 1067 (42,1) | 1258 (8,1) | 815 (4,7) | 3140 (8,8) | |
| 2019 | N | 2414 | 14 697 | 17 694 | 34 805 |
| Vaccinated (%) | 885 (36,7) | 214 (1,5) | 168 (1,0) | 1267 (3,6) | |

We found a homogeneous sex distribution in relation to the mean age (p = 0.8), PCC (p = 0.9), date of the first dose (p = 0.9), number of received doses (p = 0.8) and age group (p = 0.6).
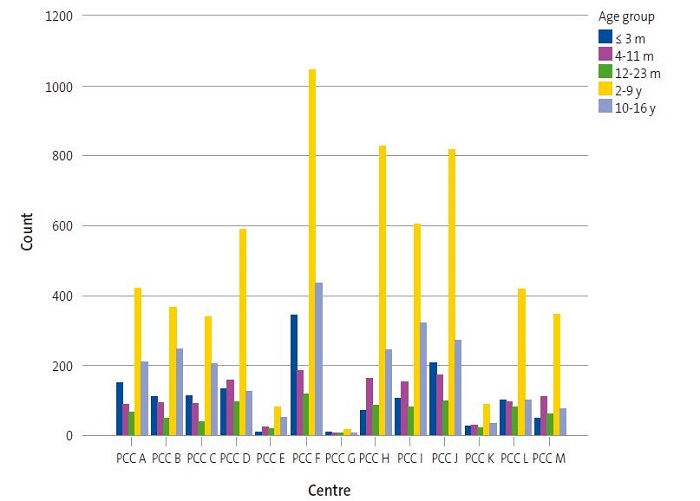
We found significant differences (p = 0.000) in the analysis of the association between PCCs and age groups (Figure 3). We found that 24.3% of patients that started vaccination at age 3 months or younger were managed at PCC F (N = 343), as were 19% of those that received the first dose in adolescence (10-16 years). Primary care centre G had one of the smallest paediatric caseloads and a higher relative frequency of vaccination in the group aged less than 24 months compared to other centres, as this age group accounted for 46.6% of total vaccinated patients in PCC G. Similarly, other centres with small paediatric caseloads, such as PCCs K, L and M had high frequencies of vaccination in patients under 2 years (36.6, 34.7 and 33.4%, respectively) compared to other age groups. In contrast, PCC B was one of the centres with the highest relative frequencies of vaccinated adolescents (28.5%) compared to other age groups.
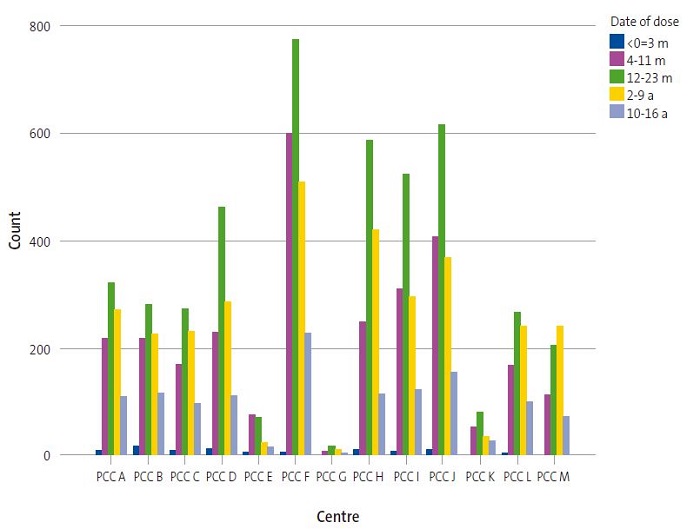
Last of all, we also found differences in vaccination rates between PCCs based on the date of vaccination (p = 0.000). In most centres, 2017 was the year with the highest vaccination rate (Figure 4). Among the most salient differences, we should highlight that in PCC E, the highest rate (41.2%) corresponded to patients that started vaccination in 2016, with the frequency of vaccination decreasing thereafter, while in PCC M, the rate kept increasing through year 2019, when 38.2% of the total vaccinations started.
| Figure 4. Distribution of paediatric patients vaccinated in the 13 primary care centres in health sector I of Zaragoza, 2015-2019 |
|---|
 |

DISCUSSION
The study was performed in a significant sample of the population of Zaragoza: 11 776 paediatric patients. Among our findings, we ought to highlight the differences in vaccination between PCCs throughout these years. The differences in vaccination rates could be due to multiple factors: the socioeconomic background of the catchment population of the centre, the rural or urban setting and even the level of motivation of health care professionals.
We found a high cumulative frequency of vaccination (32.5%), although there were significant differences between age groups. The difference in the cumulative frequency between patients aged less than 2 years and adolescents was 120.1%, with more frequent vaccination in the former. We also found opposite vaccination trends in these 2 groups: while vaccination was increasing in the group aged less than 2 years, there was a decrease in the annual vaccination rate in adolescents that was most marked in 2019. Some of the possible contributors to the difference between age groups could be that active promotion of vaccination is easier in children under 2 years due to their more frequent visits to the PCC and the higher incidence of IMD in children aged up to 2-3 years compared to adolescents. Still, adolescents are known to be a significant group of asymptomatic carriers of the pathogen. The frequency of nasopharyngeal colonization in this age group is estimated at 50%, resulting in more frequent transmission. In the case of serogroup C meningococcus, vaccination during adolescence has been found to decrease the frequency of nasopharyngeal carriage, an aspect that is currently being studied in relation to vaccination against serogroup B.3
Although at the start of vaccination active outreach to promote it was attempted in every paediatric age group, vaccination seems to have decreased considerably in older children. Our duty as paediatricians is to foster adequate communication with families in every age range to improve health literacy and promote vaccination against MenB.
Bexsero® was introduced in Spain in October 2015 as a vaccine that was not funded by the public health system for the general population. It is only publicly funded in patients with properdin or terminal complement pathway deficiency, including treatment with eculizumab, asplenia, elective splenectomy or severe spleen dysfunction, sickle cell anaemia, laboratory staff (technicians and microbiologists) and patients with a previous history of IMD.1
Although the evolution of vaccination against MenB in the cohort under study was satisfactory, vaccination rates could improve with public funding of the vaccine. The Canary Islands and Castilla y León are the only autonomous communities in Spain that fund vaccination against MenB for infants (Andalusia has just introduced funding in late 2021). Notwithstanding, the Advisory Committee on Vaccines of the Asociación Española de Pediatría (Spanish Association of Pediatrics) recommends its inclusion in routine immunization schedules nationwide. On the other hand, some European countries, such as Andorra, Italy, Ireland, Lithuania, Portugal, United Kingdom and San Marino have already included vaccination against MenB in the publicly funded routine immunization schedule.1
The United Kingdom introduced routine vaccination against MenB in 2014 and a 75% decrease in the incidence of the disease has been observed after 3 years of vaccination (95% confidence interval [95 CI]: 64 to 81).5 In a region of Quebec (Saguenay-Lac-Saint-Jean), vaccination of 83% of the population aged less than 20 years was initiated in 2014, after which there has been an observed decrease in the incidence of the disease of 86% (95 CI: 2 to 98).6 In Italy, evidence shows a vaccine effectiveness of 93.6% in Tuscany (95 CI: 55.4 to 99.1) and 91% in Veneto (95 CI: 59.9 to 97.9).7 On the other hand, Australia promotes vaccination of adolescents, and outcomes have been promising in a cohort of patients aged 16 to 19 years, with a vaccination impact of 71% (95 CI: 15 to 90) and no new cases of disease among vaccinated individuals.8 In the case of Spain, there has been a decreasing trend in the incidence of IMD caused by MenB in the past 5 years, although serogroup B continued to be the most prevalent serogroup in the 2019-2020 season. There were 91 new cases of MenB that amounted to 35% of all confirmed cases of IMD: 81.2% of cases of IMD in infants under 1 year, 71.4% of cases in children aged 1-4 years and 55.5% of cases in youth aged 15-19 years.9 Since cohort studies in other countries have evinced a high effectiveness and a significant reduction in IMD in the vaccinated population, the introduction of the MenB vaccine in the publicly funded immunization schedule of Spain is worth contemplating.
There are limitations to our study that may be relevant for the purpose of future lines of research. We do not exactly know how the SARS-CoV-2 pandemic has affected vaccination against MenB overall and in different age groups. On the other hand, it would be interested to determine the socioeconomic characteristics of each PCC and analyse the potential impact of factors such as the type of setting (urban/rural), the mean income in the catchment population or the mean frequency of visits.
CONCLUSION
There are significant differences in vaccination against serogroup B meningococcus between primary care centres, which may be due to socioeconomic and cultural differences between centres and to active outreach by health care professionals.
The vaccination coverage in infants has been increasing substantially, contrary to the adolescent age group.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose in relation to the preparation and publication of this article.
ABBREVIATIONS
IMD: invasive meningococcal disease · MenB: serogroup B meningococcus · PCC: primary care centre · 95 CI: 95% confidence interval.
REFERENCES
- Vacuna meningococo B. In: CAV-AEP [online] [accessed 22/02/2022]. Available at https://vacunasaep.org/familias/vacunas-una-a-una/vacuna-meningococo-b
- Moreno Pérez D, Álvarez García FJ, Aristegui Fernández J, Cilleruelo Ortega MJ, Corretger Rauet JM, García Sánchez N, et al. Vacunación frente al meningococo B. Posicionamiento del Comité Asesor de Vacunas de la Asociación Española de Pediatría. An Pediatr (Barc). 2015;82:198.e1-198.e9.
- Morillo Gutiérrez B, Berghezan Suárez A, Grupo de Patología Infecciosa de la AEPap. Vacunas antimeningocócicas en el adolescente: ¿por qué son importantes? Form Act Pediatr Aten Prim. 2018;11:145-52.
- Información Epidemiológica. Enfermedad Meningocócica - Epidemiología y situación mundial. In: Asociación de Médicos de Sanidad Exterior [online] [accessed 22/02/2022]. Available at www.amse.es/informacion-epidemiologica/216-enfermedad-meningococica-epidemiologia-y-situacion-mundial
- Ladhani SN, Andrews N, Parikh SR, Campbell H, White J, Edelstein M, et al. Vaccination of Infants with Meningococcal Group B Vaccine (4CMenB) in England. N Engl J Med. 2020;382:309-17.
- Deceuninck G, Lefebvre B, Tsang R, Betala-Belinga JF, De Serres G, De Wals P. Impact of a mass vaccination campaign against Serogroup B meningococcal disease in the Saguenay-Lac-Saint-Jean region of Quebec four years after its launch. Vaccine. 2019;37:4243-5.
- Azzari C, Moriondo M, NiedduF, Guarnieri V, Lodi l, Canessa C, et al. Effectiveness and Impact of the 4CMenB Vaccine against Group B Meningococcal Disease in Two Italian Regions Using Different Vaccination Schedules: A Five-Year Retrospective Observational Study (2014-2018). Vaccines (Basel). 2020;8:469.
- McMillan M, Walters l, Sullivan T, Leong LEX, Turra M, Lawrence A, et al. Impact of meningococcal B (4CMenB) vaccine on pharyngeal Neisseria meningitidis carriage density and persistence in adolescents. Clin Infect Dis. 2021;73:e99-e106.
- Enfermedad meningocócica en España, temporada 2019-2020. In: CAV-AEP [online] [accessed 22/02/2022]. Available at https://vacunasaep.org/profesionales/noticias/enfermedad-meningococica-Espana-2019-2020