Vol. 20 - Num. 80
Original Papers
Adherence to recommendations for the introduction of complementary feeding in a multicultural urban health district
Eduardo Esteban Zuberoa, Cristina Ana Baquer Sahúnb, Marta Jordán Domingob, Santiago Trueba Insab, Mónica Lubián Martínezb, Paula M.ª Barberá Pérezc, Elena Javierre Mirandad, Nuria García Sánchezd
aMIR-Medicina Familiar y Comunitaria. CS Delicias Sur. Sector Zaragoza III. Zaragoza. España.
bMIR-Medicina Familiar y Comunitaria. Sector Zaragoza III. CS Delicias Sur. Zaragoza. España.
cMIR-Pediatría. Hospital Clínico Universitario Lozano Blesa. Zaragoza. España.
dPediatra. CS Delicias Sur. Zaragoza. España.
Correspondence: E Esteban. E-mail: eezubero@gmail.com
Reference of this article: Esteban Zubero E, Baquer Sahún CA, Jordán Domingo M, Trueba Insa S, Lubián Martínez M, Barberá Pérez PM, et al. Adherence to recommendations for the introduction of complementary feeding in a multicultural urban health district. Rev Pediatr Aten Primaria. 2018;20:341-52.
Published in Internet: 12-12-2018 - Visits: 19325
Abstract
Introduction: exclusive breastfeeding is recommended for infants until age 6 months, followed by continuation of breastfeeding combined with appropriate complementary foods until age 2 years. If needed, fruits, vegetables and gluten-free cereals can be introduced between ages 4 and 6 months. There is evidence that inadequate introduction of foods may alter development and increase the risk of food allergies. The aim of our study was to assess the introduction of complementary foods in children aged less than 24 months in an urban health district with a very culturally diverse population.
Materials and methods: we conducted an observational and descriptive study through interviews conducted over a 9-month period (August 2014 to April 2015). We collected data on sociodemographic and economic variables, type of delivery, gestational age at birth, care of the infant by individuals other than the parents, vaccination and time of first visit to the primary care centre. When it came to feeding, we collected data on the mode of feeding, age at which foods other than breastmilk were introduced and the timing of introduction of complementary foods.
Results: We analysed 51 children. In 94%, complementary foods were introduced before age 6 months (fruit and gluten-free cereal). This proportion was greater in children cared for by a relative other than the parents (grandmother). We did not find statistically significant differences in any of the variables under study, except vaccination against pneumococcus (p < .001).
Conclusions: in our area, complementary feeding was introduced correctly. The implementation of community-based interventions can improve adherence to current recommendations.
Keywords
● Adherence ● Breastfeeding ● Complementary feeding ● Primary careINTRODUCTION
Complementary feeding (CF) is defined as the feeding of any type of nutrients to an infant other than those contributed by human milk. The introduction of these foods is considered an essential milestone in the care of the child, as it has a direct impact on growth, development and therefore survival.1 At present, the World Health Organization (WHO) recommends exclusive breastfeeding until age 6 months for the correct development of infants. From this age, it is important to gradually introduced healthy foods that are developmentally appropriate for the infant to continue to promote healthy growth.2 There is evidence that early introduction of CF alters nutritional and health outcomes in the infant, not only due to premature discontinuation of breastfeeding, if it happens, but also due to interferences with the adequate absorption of the nutrients in human milk, an increased incidence of food intolerance, and alterations in the development of immune mechanisms against disease that may lead to autoimmunity.3 On the other hand, late or inappropriate introduction of complementary foods may delay growth and increase the risk of malnutrition.2
In line with this evidence, current recommendations for adequate infant development are based on exclusive breastfeeding until age 6 months followed by maintenance of breastfeeding until age 2 years combined with adequate CF. In addition, the introduction of gluten-free cereals, fruits and vegetables between ages 4 and 6 months is considered appropriate in formula-fed babies.4 These recommendations have been tested in multiple studies, which have found that 800 000 children deaths per year are directly related to inadequate breastfeeding and that maintenance of breastfeeding until age 12 months is the best strategy to decrease mortality in the under-5 population.5,6 Globally, 9.5 million children aged less than 5 years die every year, and 35% of these deaths can be attributed to malnutrition.7
This problem is particularly relevant in developing countries, where it is estimated that 19.4% of children aged less than 5 years are underweight and 29.9% exhibit stunting.8 There is evidence of a direct correlation between variables like parental educational attainment and skills and the dietary habits of their children.9,10 Socioeconomic status11 and employment of both parents in a household, which limits the time available to prepare food and is associated with increased consumption of processed foods,12 have also been described as independent variables that influence infant nutrition practices. In developed countries, these factors have contributed to the early introduction of CF, which has caused an increase in the prevalence of excess weight in the population aged less than 1 year from 1.8 to 33.9%.13 This, in turn, has been associated with increases in the incidence of gastrointestinal problems and respiratory tract infections and in the risk of atopy and allergies.14
Several studies have explored how to improve adherence to recommendations for the introduction of CF. The strategies employed in these studies have ranged from educating mothers on how to promote the development of healthy dietary habits to the use of fortified foods or micronutrient supplements, and were proven to have an impact on weight gain and developmental outcomes.15-17
The aim of our study was to evaluate the introduction of CF in children aged less than 24 months managed in an urban primary care centre in a developed country (Spain) with a culturally diverse catchment population. To do so, we analysed variables such socioeconomic status, employment, educational attainment, country of origin and role of the primary caregiver (parent, other relative, child care worker) to determine whether they influenced the introduction of CF.
MATERIALS AND METHODS
We conducted an observational descriptive study in which we collected data for a 9-month period (August 2014-April 2015) by holding face-to-face interviews with the parents of children aged 6 to 24 months that visited the paediatrics clinic of the Delicias Sur Primary Care Centre of Zaragoza (Spain). The data were anonymised, and the paediatrician managing the child was not involved in the interview or its results to prevent response bias.
We collected data for sociodemographic variables (parental age, country of origin, number of previous children), type of delivery (vaginal, assisted or caesarean), gestational age at birth, parental socioeconomic variables (smoking, employment status, educational attainment, socioeconomic status associated with the coverage category stated in their health card, personal history of being breastfed, and having been granted a leave or a reduced work schedule after childbirth), care of the infant by other members of the family (grandmother) or in child care facilities, administration of vaccines not included in the official, publicly funded immunization schedule of the Autonomous Community of Aragon (pneumococcal, varicella and rotavirus) and the timing of the first visit to the primary care centre.
Coverage in the Spanish health care system is stratified into 5 categories whose codes appear in the individual health card of the user (tarjeta sanitaria individual [TSI]): TSI code 001 for exempt individuals and individuals receiving tax-free pensions or with long-term unemployment (exempt from copay); TSI code 002 for pensioners (reduced drug copay of 10%); TSI code 003 for actively employed citizens with incomes of up to 18 000 euro (copay of 40%); TSI code 004 for actively employed citizens with incomes between 18 000 and 100 000 euro (copay of 50%); TSI code 005 for actively employed individuals and pensioners with incomes of more than 100 000 euro (copay of 60%).
In terms of nutrition, we analysed the initial mode of feeding in the infant (exclusive or mixed breastfeeding, artificial feeding), the timing of initiating a mode of feeding other than breastfeeding (before or after age 1 month), and age (in months) of introduction of the different complementary foods (gluten-free cereal, cereal with gluten, fruit, vegetables, meat, white fish, oily fish, egg yolk, whole egg, legumes, dairy products [infant yogurt, plain yogurt, flavoured yogurt, fresh cheese and other processed liquid milk-based products] and other products such as snacks, sweets, chocolates and processed prepared foods). For the purpose of the study, we applied the guidelines for the correct introduction of CF of the Asociación Española de Pediatría de Atención Primaria (Spanish Association of Primary Care Paediatrics).18
We performed an initial descriptive analysis, followed by a bivariate analysis for comparing variables. We used the χ2 to compare qualitative variables and the Student t test to compare quantitative variables. We defined statistical significance as p <0.005. We performed the statistical analyses with the software IBM SPSS® Statistics version 20.
RESULTS
We analysed a total of 51 children aged 6 to 24 months. Of the entire sample, a total of 37% of children (n = 19) had been born in Spain. The mean age (± standard deviation [SD]) of mothers and fathers was 31.24 ± 6.39 and 36.3 ± 8.15 years, respectively. The mean number of previous children (± SD) was 0.56 ± 0.185 in Spanish participants, compared to 0.87 ± 0.218 in non-Spanish participants. The mean days (± SD) elapsed from birth to the time of the first visit to the paediatrician was 6.68 ± 0.593 in the Spanish subgroup and 11.56 ± 3.561 in the non-Spanish subgroup. In this sample, 78.4% of the children were born through normal vaginal delivery (n = 40), 19.6% by caesarean section (n = 10) and 2% through assisted vaginal delivery (n = 1). When it came to socioeconomic status, which we assessed based on the TSI code, 5.9% of the sample were in the 001 category (n = 3), 2% in category 002 (n = 1), 76.5% in category 003 (n = 39) and 15.7% in category 004 (n = 8). We did not find statistically significant differences in these variables between Spanish and non-Spanish participants (p <.005).
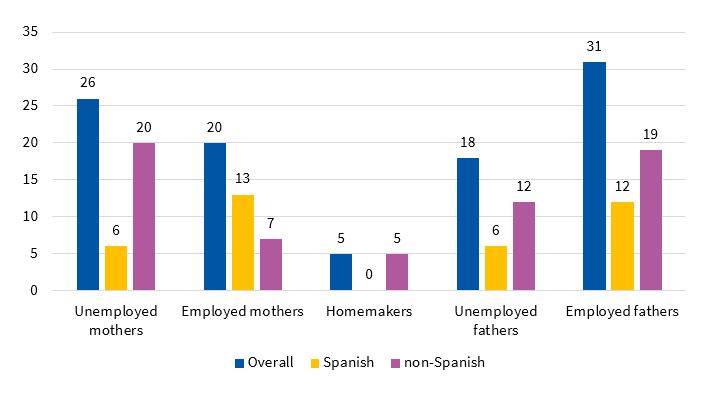
The percentage of mothers and fathers that were unemployed was 51% and 36.7%, respectively (n = 26 and n = 18). In the subgroup of Spanish mothers (n = 19), 68.42% were actively employed (n = 13) and 31.58% were unemployed (n = 6). Of the non-Spanish mothers (n = 32), 21.87% were employed (n = 7) and 62.5% unemployed (n = 20); 15.62% (n = 5) were homemakers. Of all Spanish fathers (n = 18), 66.66% were employed (n = 12) and 33.33% unemployed (n = 6). In non-Spanish fathers (n = 31), the percentages were 61.33% (n = 19) and 38.7% (n = 12), respectively (Figure 1). The statistical significance of these comparisons between the Spanish subgroup and the rest of the sample was not assessable.
| Figure 1. Employment status of parents, overall and by country of origin |
|---|
 |

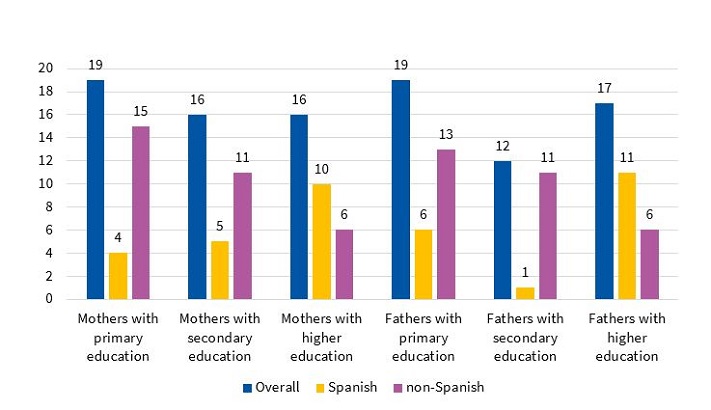
Educational attainment was similar in mothers and fathers. Of the Spanish mothers (n = 19), 21% (n = 4) had completed primary education, 26.31% (n = 5) secondary education, and 52.63% (n = 10) higher education degrees. In non-Spanish mothers (n = 32), the corresponding figures were 46.87% (n = 15), 34.37% (n = 11) and 18.75% (n = 6), respectively. Of the Spanish fathers (n = 18), 33.33% had completed primary education (n = 6), 5.55% secondary education (n = 1), and 61.11% higher education (n = 11). In non-Spanish fathers (n = 30), the corresponding figures were 43.33% (n = 13), 36.66% (n = 11) and 20% (n = 6), respectively (Figure 2). The statistical significance of these comparisons between the Spanish subgroup and the rest of the sample was not assessable.
| Figure 2. Educational attainment of parents, overall and by country of origin |
|---|
 |

As for tobacco exposure, 9.8% of mothers smoked (n = 5, 1 Spanish and 4 non-Spanish) and 90.2% did not (n = 46, 18 Spanish and 28 non-Spanish). Among the men, 22% smoked (n = 11, 6 Spanish and 5 non-Spanish) and 78% did not (n = 40, 13 Spanish and 27 non-Spanish). We could not perform an inferential analysis of this variable. Of all interviewed mothers, 90.19% had been breastfed (n = 46). In the analysis by country of origin, we found that 84.21% of Spanish mothers (n = 16) had been breastfed during infancy, compared to 93.75% of non-Spanish mothers (n = 30). The statistical significance of these comparisons was also not assessable.
When we analysed work benefits granted at the time of child birth, we found that paid maternity leave was granted to 43.13% of mothers; which, broken down by country of origin, corresponded to 57.89% of Spanish mothers (n = 11) and 34.37% of non-Spanish mothers (n = 11). In addition, 11.76% of mothers chose the option of working reduced hours (n = 6), of who 66.66% (n = 4) were Spanish and 33.33% (n = 2) not Spanish. An unpaid leave was granted to 3.92% of mothers (n = 2), 1 Spanish mother and 1 non-Spanish mother. The statistical significance of these comparisons was not assessable.
As for the mode of feeding, 74.5% of infants (n = 38) were breastfed, 13.72% (n = 7) received mixed feeding and 11.76% (n = 6) were formula-fed. The statistical significance of this comparison was also not assessable.
Our analysis of vaccination revealed statistically significant differences between the Spanish and the non-Spanish subgroups in the administration of the pneumococcal vaccine (p < .001). In our sample, 47.05% of the children had received this vaccine (n = 24). In the Spanish subgroup, this preventive measure had been implemented in 78.94% of infants (n = 15), while in the non-Spanish subgroup the vaccine coverage amounted to only 28.12% (n = 9). None of the children had received the varicella vaccine. When it came to the rotavirus vaccine, we found no statistically significant differences based on country of origin (p > .005): overall, 27.45% of children had received it (n = 14), corresponding to 47.36% of Spanish children (n = 9) and 15.62% of non-Spanish children (n = 5).
In total, 11.75% of children were enrolled in child care centres (n = 6), of who half were Spanish (n = 3). Analysing this by subgroup, this percentage included 15.78% of Spanish children (n = 3) and 9.37% of non-Spanish children (n = 3). The mean age at initial enrolment was 5.83 ± 0.75 months. We were also unable to assess the statistical significance of this variable. We did, however, find a statistically significant association between care by another (grandparent) and country of origin. Of all children, 43.13% (n = 22) were in the care of a grandparent. Broken down by country of origin, this corresponded to 73.68% of Spanish infants (n = 14) and 25% of non-Spanish infants (n = 8). The mean age at the beginning of this care arrangement was 1.45 ± 1.01 months.
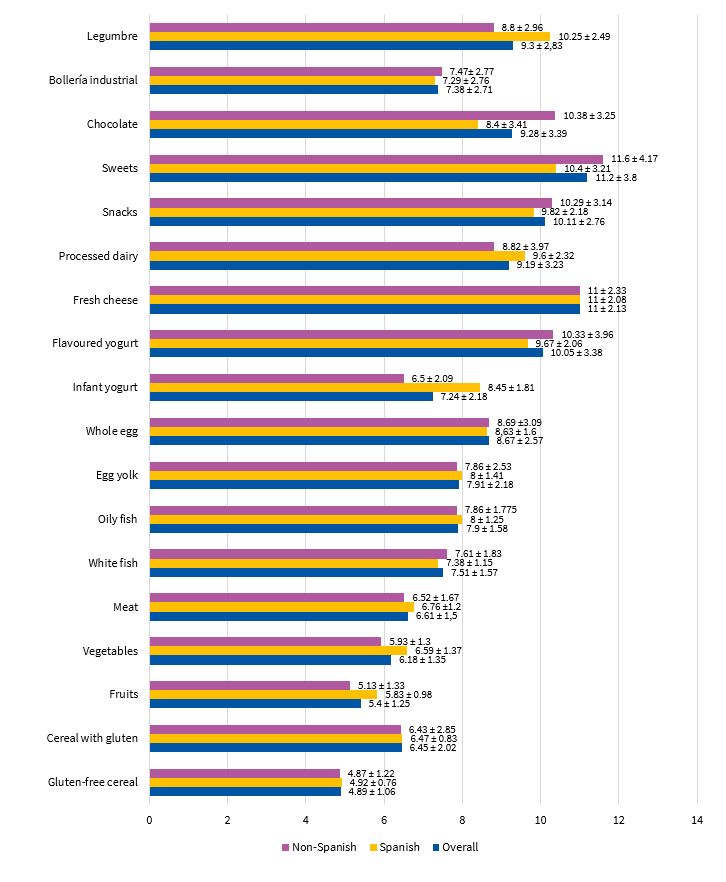
The most salient findings of our analysis of the introduction of complementary foods were the introduction of gluten-free cereals at a mean age (± SD) of 4.89 ± 1.06 months and of fruit at 5.4 ± 1.25 months. Other foods worth highlighting were processed milk-based drinks (age 9.19 ± 3.23 months), snacks (age 10.11 ± 2.76 months), sweets (age 11.2 ± 3.8 months), chocolate (9.28 ± 3.39) and processed baked goods (age 7.38 ± 2.71 months). These data show that 70.58% of children in the sample had consumed gluten-free cereals before age 6 months. When it came to fruit, the proportion rose to 94.11%. Processed snacks, mil-based drinks, chocolate and baked goods were introduced before age 12 months in 45% of children and after that age in 11%, and had not yet been introduced in 44% of cases. Figure 3 presents detailed information about the age at which the different complementary foods were introduced.
| Figure 3. Age at time of introduction of each type of food (mean ± standard deviation) |
|---|
 |

DISCUSSION
In our study, conducted in a primary care centre with a very diverse catchment population due to the multiple nationalities represented in the population that resides in our health district, we sought to assess socioeconomic variables, parental educational attainment and the ages at which complementary foods were introduced. Our goal was to determine whether there is an association between these variables for the purpose of performing a second study in which to explore interventions aimed at each subset of the population. The collected data showed that CF was introduced before age 6 months in 94.11% of the children in the sample (mainly through the introduction of fruit). We also found that foods such as artificial milk-based drinks, snacks, sweets, chocolate and processed baked goods were introduced early, which contravenes the current guidelines for CF in Spain, which are based on the gradual introduction of health foods starting at 6 months, preceded by exclusive breastfeeding.19 While in certain subsets of the population infants may require CF before age 6 months (infants fed formula or a combination of formula and breastmilk), current guidelines do include the possibility of introducing vegetable, cereals or fruits between ages 4 and 6 months under these circumstances.18,19 We did not find statistically significant differences in these patterns in the sample under study based on country of origin or socioeconomic status.
There are many studies in the medical literature on the subject of infant nutrition, especially in developing countries. We cannot directly compare these studies to ours due to social, demographic and cultural differences, but they can help us identify common trends. Thus, there is evidence of an early introduction of rice in certain Asian regions, which can be attributed to cultural factors.20 In Brazil, a prospective study with a 10-year followup found that adherence to CF guidelines increased with increasing parental educational attainment. However, the data still showed that fruits, meats, vegetables, cow’s milk and processed food were introduced too early, while breastfeeding was discontinued too soon. These outcomes were not associated with socioeconomic status, and have been attributed to the scarcity of educational interventions for future parents at the primary care level.2,21,22 The findings of that study differed from those of other studies conducted in countries like Nicaragua, where there was evidence of consumption of a broader variety of foods in children of parents with higher socioeconomic status and educational attainment, including snacks and highly-processed foods.23 Other studies have also found an association between the correct introduction of CF and higher socioeconomic status and educational attainment.24,25
A higher parental educational attainment and the implementation of health education interventions in developing countries such as Nepal,26 Indonesia27, Pakistán28, India29, Tanzania30, Uganda31, Ethiopía32, Nigeria33 and Ghana34 have been associated with increased adherence to the general recommendations of the WHO on the foods to be given based on the age of the child and the number of feedings needed per day, leading to improved nutritional outcomes.35
In developed countries, the correct introduction of CF is associated with greater maternal age, higher socioeconomic status, multiparity and lower body mass index.36,37 We ought to highlight the higher rate of breastfeeding observed in these studies compared to ours (97% vs. 74.5%), which in turn is associated with a later and more adequate introduction of CF.36 On the other hand, an early return to work and care by a relative other than the parents have been associated with a shorter duration of breastfeeding and earlier introduction of CF.38,39 These factors may have contributed to the early introduction of certain complementary foods, like fruits and gluten-free cereals, and the use of processed foods observed in our study, as we found lower breastfeeding rates in the mothers in our sample, which may have been due to fewer mothers getting maternity leave or a reduction of their work schedule and to the significant percentage of children cared for by grandparents, which was statistically significant in the Spanish subset of the sample.
A study conducted in Mexico found improvement in the introduction of CF in recent years, although poor practices still persist.40 The study found earlier introduction of fruits and vegetables (at around age 4 months) and of meat (age 6 months) compared to our cohort. We ought to note that early introduction of CF in the Mexican study was less frequent in families of lower socioeconomic status, which is inconsistent with our findings and those of similar studies conducted later on.36,37 In more developed countries, such as Australia, more than 75% of infants consume fruits, vegetables and gluten-free cereals and about 25% consume meat by age 6 months.41 We ought to note that one of the main reasons given by respondents in our study for introducing complementary foods was that the child expressed an interest in trying them. The trends observed in the Australian study were more similar to those found in our sample, although vegetables were introduced earlier in Australia. Studies conducted in Northern Europe found greater adherence to CF guidelines compared to Spain, and we found that poor adherence with recommendations was associated with an increased risk of childhood obesity.42
A review of studies similar to ours conducted in developed countries revealed that there is greater concern with “poor growth” in infants in the immigrant population, which in a study was unexpectedly combined with the early introduction of manufactured baked goods.43 These findings were not consistent with ours, as we did not find differences in the probability of this practice based on country of origin. The data also revealed similar patterns between the native and immigrant subgroups in the introduction of fruits, vegetables, yogurt and cereals, which was consistent with our findings. However, this is inconsistent with the results of other studies that have found that immigrant status was a protective factor for prolonged breastfeeding and later introduction of CF.44 Multinational European studies have found similar trends in the introduction of CF.45 The authors reported that 37.2% of formula-fed infants were fed complementary foods before age 4 months. The proportion was lower in breastfed infants (17.2%). The proportions found in Spain were close to the observed averages for Europe, with a protective effect of higher socioeconomic status and educational attainment. The evidence from Mediterranean countries, such as Italy, showed that CF had already been introduced by age 6 months in 94% of infants,46which is consistent with our results.
There is evidence that in the early stages of introducing solids, the greatest concern of parents is the healthy development of the child, and they are aware that there is a right time to introduce each food and that the diet of infants is different from the diet of the parents. This changes in later stages (12 months) in which the development of the child is perceived as a long-term process and priority is given to the child’s integration in the family and social environment, which may lower the priority of feeding the child appropriate foods.47 This is the reason that this study emphasised that educational interventions for CF need to be prolonged and not limited to the early stages of its introduction. This behavioural pattern may explain to some extent the feeding of chocolate, snacks and processed dairy early in the child’s development, as we observed in our study.
As for the outcomes of this pattern, there is evidence that the premature introduction of CF (before age 4 months or even between 4 and 6 months) increases the risk of childhood obesity, with the associated impact on health and health care costs. 13 In Spain, different studies have compared the efficacy of various interventions to improve adherence to recommendations. This research has shown that the use of written materials is more frequent in mothers who are older and of higher socioeconomic status, and is associated with longer duration of breastfeeding and adequate introduction of CF.48,49 With the advent of new technologies, evidence has emerged that low-intensity interventions in written form delivered electronically, such as text messages, can also be useful.50 The study that analysed this strategy showed that it increased the proportion of mothers that exclusively breastfeed their infants until age 6 months and was associated with the correct introduction of CF. On the other hand, face-to-face interactions, even with health professionals, appear to be less effective.48,49,51 For this reason, several studies propose focusing higher-intensity interventions in younger mothers of lower socioeconomic status.52
One of the limitations of our study is its small sample size, which may hinder the accurate assessment of the statistical significance of variables under study. We also did not assess the impact of current interventions in our area in the medium and long term, such as the incidence of childhood obesity. In addition, the information provided by parents about the introduction of CF in the older children in our sample may have been less accurate due to the time elapsed between the introduction of foods and the interview. The strengths of our study are the cultural and socioeconomic diversity of our sample, which allowed us to identify trends in each subpopulation that could help fit future strategies better to target specific needs. Furthermore, the fact that the data were not collected by the paediatrician added a degree of confidentiality that may have prevented response bias.
In conclusion, in our region CF is introduced before age 6 months in 94% of children, which contravenes the recommendations of maintaining exclusive breastfeeding until age 6 months but adheres with the recommendation of introducing CF (fruits, vegetables and gluten-free cereals) between ages 4 and 6 months in case the infant is formula-fed or fed a combination of breast milk and formula and requires complementary foods. We also ought to highlight the excessive consumption of processed foods like snacks, chocolates, sweets and processed dairy, which was independent of country of origin. We found a correlation between the primary caregiver being a relative other than the parents (grandmother) and low adherence to dietary recommendations, which may result from parental challenges in balancing work and child care. Although we did not find evidence of it in our study, a high socioeconomic status and educational attainment usually perform as protective factors that promote correct nutrition of the child. Interventions targeting parents to improve the introduction of CF and promote BF have proven effective, so it is important to promote and encourage long-term BF maintenance. The implementation of these measures may be facilitated by emerging technologies, which make it easier to deliver interventions with no associated reduction in effectiveness. Further research is required to better define the variables that influence the introduction of CF for the purpose of developing specific intervention programmes targeting each subset of the population.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article.
ABBREVIATIONS
CF: complementary feeding · SD: standard deviation · WHO: World Health Organization.
REFERENCES
- Giugliani ERJ, Victora CG. Alimentação complementar. J Pediatr (Rio J). 2000;76:253-62.
- Oliveira DA, Castro IR, Jaime PC. Complementary feeding patterns in the first year of life in the city of Rio de Janeiro, Brazil: time trends from 1998 to 2008. Cad Saude Publica. 2014;30:1755-64.
- Van Odjik J, Kull I, Borres MP. Breastfeeding and allergic disease: a multidisciplinary review of the literature (1966-2001) on the mode of early feeding and its impact on later atopic manifestations. Allergy. 2003;58:833-43.
- Vossenaar M, van Beusekom I, Doak C, Solomons NW. Feeding patterns before 6 months of age: the relative validity of recall from interviews of mothers of Guatemalan infants and toddlers. Asia Pac J Clin Nutr. 2014;23:634-40.
- Jones G, Steketee RW, Black RE, Bhutta ZA, Morris SS. How many child deaths can we prevent this year? Lancet. 2003;9377:65-71.
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;9890:427-51.
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969-87.
- Stevens GA, Finucane MM, Paciorek CJ, Flaxman SR, White RA, Donner AJ, et al. Trends in mild, moderate, and severe stunting and underweight, and progress towards MDG 1 in 141 developing countries: a systematic analysis of population representative data. Lancet. 2012;380:824-34.
- Patel A, Pusdekar Y, Badhoniya N, Borkar J, Agho KE, Dibley MJ. Determinants of inappropriate complementary feeding practices in Young children in India: secondary analysis of National Family Health Survey 2005-2006. Matern Child Nutr. 2012;8:28-44.
- Senarath U, Godakandage SS, Jayawickrama H, Siriwardena I, Dibley MJ. Determinants of inappropriate complementary feeding practices in Young children in Sri Lanka: secondary data analysis of Demographic and Health Survey 2006-2007. Matern Child Nutr. 2012;8:60-77.
- Joshi N, Agho KE, Dibley MJ, Senarath U, Tiwari K. Determinants of inappropriate complementary feeding practices in young children in Nepal: secondary data analysis of Demographic and Health Survey 2006. Matern Child Nutr. 2012;8:45-59.
- Jabs J, Devine CM. Time scarcity and food choices: an overview. Appetite. 2006;47:196-204.
- Pearce J, Taylor MA, Langley-Evans SC. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:1295-306.
- Seach KA, Dharmage SC, Lowe AJ, Dixon JB. Delayed introduction of solid feeding reduces child overweight and obesity at 10 years. Int J Obes. 2010;34:1475-9.
- Caulfield LE, Huffman SL, Piwoz EG. Interventions to improve intake of complementary foods by infants 6 to 12 months of age in developing countries: impact on growth and on the prevalence of malnutrition and potential contribution to child survival. Food Nutr Bull. 1999;20:183-200.
- Dewey KG, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008;4(Suppl 1):24-85.
- Imdad A, Yakoob MY, Bhutta ZA. Impact of maternal education about complementary feeding and provision of complementary foods on child growth in developing countries. BMC Public Health. 2011;11S25.
- Promoción de la lactancia materna. En: Asociación Española de Pediatría de Atención Primaria (ed.). Programa de Salud Infantil. Madrid: Exlibris; 2009. p. 215-36.
- Agostoni C, Decsi T, Fewtrell M, Goulet O, Kolacek S, Koletzko B, et al. Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2008;46:99-110.
- Inoue M, Binns CW. Introducing solid foods to infants in the Asia Pacific region. Nutrients. 2014;6:276-88.
- Souza FI, Caetano MC, Ortiz TT, Silva SG, Sarni RO. Complementary feeding of infants in their first year of life: focus on the main pureed baby foods. Rev Assoc Med Bras. 2014;60:231-5.
- Souza JP, Prudente AM, Silva DA, Pereira LA, Rinaldi AE. Evaluation of employees in public day care centers knowledge about breastfeeding and complementary feeding. Rev Paul Pediatr. 2013;31:480-7.
- Contreras M, Blandón EZ, Persson LÅ, Hjern A, Ekström EC. Socio-economic resources, young child feeding practices, consumption of highly processed snacks and sugar-sweetened beverages: a population-based survey in rural northwestern Nicaragua. BMC Public Health. 2015;15:25.
- Senarath U, Agho KE, Akram DE, Godakandage SS, Hazir T, Jayawickrama H, et al. Comparisons of complementary feeding indicators and associated factors in children aged 6-23 months across five South Asian countries. Matern Child Nutr. 2012;8:89-106.
- Victor R, Baines SK, Agho KE, Dibley MJ. Factors associated with inappropriate complementary feeding practices among children aged 6-23 months in Tanzania. Matern Child Nutr. 2014;10:545-61.
- Khanal V, Sauer K, Zhao Y. Determinants of complementary feeding practices among Nepalese children aged 6-23 months: findings from Demographic and Health Survey 2011. BMC Pediatr. 2013;13:131.
- Fahmida U, Kolopaking R, Santika O, Sriani S, Umar J, Htet MK, et al. Effectiveness in improving knowledge, practices, and intakes of “key problem nutrients” of a complementary feeding intervention developed by using linear programming: experience in Lombok, Indonesia. Am J Clin Nutr. 2015;101:455-61.
- Saleem AF, Mahmud S, Baig-Ansari N, Zaidi AK. Impact of maternal education about complementary feeding on their infants’ nutritional outcomes in low- and middle-income households: a community-based randomized interventional study in Karachi, Pakistan. J Health Popul Nutr. 2014;32:623-33.
- Kushwaha KP, Sankar J, Sankar MJ, Gupta A, Dadhich JP, Gupta YP, et al. Effect of peer counselling by mother support groups on infant and young child feeding practices: the Lalitpur experience. PLoS One. 2014;9:e109181.
- Kulwa KB, Verstraeten R, Bouckaert KP, Mamiro PS, Kolsteren PW, Lachat C. Effectiveness of a nutrition education package in improving feeding practices, dietary adequacy and growth of infants and young children in rural Tanzania: rationale, design and methods of a cluster randomised trial. BMC Public Health. 2014;14:1077.
- Ickes SB, Hurst TE, Flax VL. Maternal literacy, facility birth, and education are positively associated with better infant and young child feeding practices and nutritional status among ugandan children. J Nutr. 2015;145:2578-86.
- Negash C, Belachew T, Henry CJ, Kebebu A, Abegaz K, Whiting SJ. Nutrition education and introduction of broad bean-based complementary food improves knowledge and dietary practices of caregivers and nutritional status of their young children in Hula, Ethiopia. Food Nutr Bull. 2014;35:480-6.
- Balogun TB, Yakubu AM. Recent illness, feeding practices and father’s education as determinants of nutritional status among preschool children in a rural Nigerian community. J Trop Pediatr. 2015;61:92-9.
- Issaka AI, Agho KE, Burns P, Page A, Dibley MJ. Determinants of inadequate complementary feeding practices among children aged 6-23 months in Ghana. Public Health Nutr. 2015;18:669-78.
- Lassi ZS, Das JK, Zahid G, Imdad A, Bhutta ZA. Impact of education and provision of complementary feeding on growth and morbidity in children less than 2 years of age in developing countries: a systematic review. BMC Public Health. 2013;13:S13.
- Betoko A, Charles MA, Hankard R, Forhan A, Bonet M, Saurel-Cubizolles MJ, et al. Infant feeding patterns over the first year of life: influence of family characteristics. Eur J Clin Nutr. 2013;67:631-7.
- Kronborg H, Foverskov E, Væth M. Breastfeeding and introduction of complementary food in Danish infants. Scand J Public Health. 2015;43:138-45.
- Visness CM, Kennedy KI. Maternal employment and breast-feeding: findings from the 1988 National Maternal and Infant Health Survey. Am J Public Health 1997;87:945-50.
- Kim J, Peterson KE. Association of infant child care with infant feeding practices and weight gain among US infants. Arch Pediatr Adolesc Med 2008;162:627-33.
- González de Cossío T, Escobar-Zaragoza L, González-Castell D, Reyes-Vázquez H, Rivera-Dommarco JA. Breastfeeding in Mexico was stable, on average, but deteriorated among the poor, whereas complementary feeding improved: results from the 1999 to 2006 National Health and Nutrition Surveys. J Nutr. 2013;143:664-71.
- Newby RM, Davies PS. A prospective study of the introduction of complementary foods in contemporary Australian infants: What, when and why? J Paediatr Child Health. 2015;51:186-91.
- Imai CM, Gunnarsdottir I, Thorisdottir B, Halldorsson TI, Thorsdottir I. Associations between infant feeding practice prior to six months and body mass index at six years of age. Nutrients. 2014;6:1608-17.
- Van Eijsden M, Meijers CM, Jansen JE, de Kroon ML, Vrijkotte TG. Cultural variation in early feeding pattern and maternal perceptions of infant growth. Br J Nutr. 2015;114:481-88.
- Castro PD, Layte R, Kearney J. Ethnic variation in breastfeeding and complimentary feeding in the Republic of Ireland. Nutrients. 2014;6:1832-49.
- Schiess S, Grote V, Scaglioni S, Luque V, Martín F, Stolarczyk A, et al. Introduction of complementary feeding in 5 European countries. J Pediatr Gastroenterol Nutr. 2010;50:92-8.
- Pani P, Carletti C, Knowles A, Parpinel M, Concina F, Montico M, et al. Patterns of nutrients’ intake at six months in the northeast of Italy: a cohort study. BMC Pediatr. 2014;14:127.
- Nielsen A, Michaelsen KF, Holm L. Parental concerns about complementary feeding: differences according to interviews with mothers with children of 7 and 13 months of age. Eur J Clin Nutr. 2013;67:1157-62.
- Gage H, Williams P, Von Rosen-Von Hoewel J, Laitinen K, Jakobik V, Martín-Bautista E, et al. Influences on infant feeding decisions of first-time mothers in five European countries. Eur J Clin Nutr. 2012;66:914-9.
- Hunsberger M, Lanfer A, Reeske A, Veidebaum T, Russo P, Hadjigeorgiou C, et al. Infant feeding practices and prevalence of obesity in eight European countries - the IDEFICS study. Public Health Nutr. 2013;16:219-27.
- Jiang H, Li M, Wen LM, Hu Q, Yang D, He G, et al. Effect of short message service on infant feeding practice: findings from a community-based study in Shanghai, China. JAMA Pediatr. 2014;168:471-8.
- Škledar MT, Milošević M. Breastfeeding and time of complementary food introduction as predictors of obesity in children. Cent Eur J Public Health. 2015;23:26-31.
- Caroli M, Mele RM, Tomaselli MA, Cammisa M, Longo F, Attolini E. Complementary feeding patterns in Europe with a special focus on Italy. Nutr Metab Cardiovasc Dis. 2012;22:813-18.





