Vol. 20 - Num. 80
Original Papers
Nutrient composition and sugar content of dairy products targeting young children in supermarkets
Ana Frades Payoa, Miguel Ángel Royo Bordonadab
aVeterinaria de Equipo de Atención Primaria. Servicio Extremeño de Salud. Badajoz. España.
bInstituto de Salud Carlos III. Madrid. España.
Correspondence: A Frades. E-mail: anafradesp@gmail.com
Reference of this article: Frades Payo A, Royo Bordonada MA. Nutrient composition and sugar content of dairy products targeting young children in supermarkets. Rev Pediatr Aten Primaria. 2018;20:353-63.
Published in Internet: 27-11-2018 - Visits: 37753
Abstract
Objectives: to analyse the nutrient composition, free sugar content and display of health claims or endorsements from scientific or health organizations of growing-up milks made for children aged 1 to 3 years. Also, to compare their nutrient composition to the composition of whole cow’s milk.
Methods: descriptive study of a sample of 20 growing-up milks sold in supermarkets in Badajoz (Spain). We obtained data on their nutrient composition and any claims or endorsements from the product labels. We estimated the free sugar content of the products by subtracting the total sugar content in cow’s milk from the total sugar content reported in the labels.
Results: compared to whole cow’s milk, growing-up milks had a higher energy content (67.7 vs. 65 kcal/100 ml) and carbohydrate content (47.5% vs. 29%), and a lower fat content (37.7% vs. 52%) and protein content (14.8% vs. 19%). Free sugars contributed between 3% and 22% of the total energy content. All products featured some type of claim, and 60% had unauthorised health claims and endorsements from the Asociación Española de Pediatría.
Conclusions: the high content of added sugars in growing-up milks contravenes de recommendations of the World Health Organization and nutrition experts. Therefore, their nutrient composition should be subject to regulation, as well as their labelling, preferably with an interpretive format, while claims or endorsements should only be allowed in products that adhere to nutritional recommendations.
Keywords
● Children ● Growing-up milk ● Labelling ● Marketing ● Obesity ● SugarINTRODUCTION
In Spain, about 1 in 3 children have overweight or obesity, one of the highest proportions in Europe.1 Although the prevalence of childhood obesity seems to have stabilised in recent years, the percentage of parents that perceive their children’s excess weight incorrectly continues to grow at an alarming rate.2 Among the various factors that contribute to this childhood obesity epidemic, sugary drinks are associated with an increased risk from early childhood.3 Furthermore, excessive consumption of added sugars by children is also associated with tooth decay and several cardiovascular risk factors, such as high blood pressure, hypercholesterolaemia and diabetes mellitus type 2.4
Consumption of sweetened foods from early ages can foster a preference for sugar in foods that persists through adolescence, which contributes to the development and maintenance of unhealthy habits.5 Experimental studies in rodents have demonstrated that consumption of sugar activates a metabolic feedback mechanism in the reward system of the brain.6 Similarly, some individuals may become addicted to sugar in stressful situations through the activation of reward pathways in the brain, making them more vulnerable to obesity and other diseases associated with the consumption of sugar.6
The World Health Organization (WHO) recommends exclusive breastfeeding from 1 hour of life to age 6 months, followed by the progressive introduction of a variety of nutritious complementary foods with no added salt or sugar while still maintaining breastfeeding.7,8 Furthermore, it recommends limiting consumption of free sugars to a maximum of 10% of the total energy intake, while noting that keeping consumption below 5% has added benefits, especially in the prevention of dental caries.9-12 The committees on nutrition of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition and the Asociación Española de Pediatría Extrahospitalaria y Atención Primaria (Spanish Association of Outpatient and Primary Care Paediatrics) recommend consumption of whole milk in children aged less than 2 years and 2 to 3 servings of dairy products in preschool- and school-aged children.13,14
In recent years, dairy products targeted to very young children (1-3 years) have entered the market, drinks made with cow’s milk and enriched with various micronutrients and described as “growing-up milk” or “toddler” dairy drinks/products, designations that are not specifically regulated by European Union law. Despite the recommendations of the WHO and expert committees on nutrition, and contrary to infant and follow-up formulas, these drinks, which we will refer to as “growing-up milk” (GUM), usually contain variable amounts of added sugars.15
Objectives
Our aim was to analyse the nutrient composition and free sugar content of GUMs marketed for children aged 1 to 3 years and to compare it with those of whole cow’s milk. In addition, we assessed the presence of health or nutritional claims and endorsements from scientific or health institutions in the labelling.
MATERIALS AND METHODS
Study design and sample
We conducted a descriptive study of the sugar content of GUMs marketed for young children (aged 1 to 3 or more years) in the supermarkets of the city of Badajoz (Spain). To obtain the sample for study, we visited local stores of large, nationwide supermarket chains (Hipercor®, Carrefour®, Mercadona®, Aldi®, LIDL®, Supermercados El Corte Inglés®, Carrefour® Express and DIA®). In each site, we went through the child nutrition and dairy product aisles, and overall we found 20 different types of GUM of 6 different brands (Puleva®, Nestlé®, Hacendado®, Celta®, Kaiku® and DIA®) targeted to children aged 1 to 3 years. These 20 products constituted the study sample.
Data collection and outcome variables
We obtained data on the energy content and total sugar and added sugar contents of each selected products from the label provided by the manufacturer in the packaging of the products available for sale. The WHO defines free sugars as “all monosaccharides and disaccharides added to foods by the manufacturer, cook or consumer, plus sugars naturally present in honey, syrups and fruit juices.”16 This definition of free sugars does not include sugars that, although not added, may have been released due to a manufacturing process.17 To detect the presence of added sugars, we reviewed the list of ingredients and collected how they were labelled in each case. We obtained the total sugar content per 100 ml of product from the nutrition facts table. Since the labels do not specify the amount of added sugar, to estimate the amount of free sugars (those that are not naturally present in milk), we calculated the difference between the total sugar in the label and the sugar content of low-fat or fat-free cow’s milk (4.6 g/100 g), the milks used in the manufacturing of GUMs, which we obtained from the information available in the Spanish Food Composition Database (Base de Datos Española de Composición de Alimentos [BEDCA], www.bedca.net). Since we were unable to determine the actual percentage of milk contained by each product (a datum that was not provided in the label), we made a conservative estimate of the free sugars that should be interpreted as a lower limit in cases where the sole 2 ingredients in one product were cow’s milk and added sugars.
We documented the presence of health or medicinal property claims and of endorsements from public health or scientific institutions in labels or in the packaging of products sold in sets of several units. Regulation (EC) 1924/2006 defines “health claim” as any claim that states, suggests or implies that a relationship exists between a food category, a food or one of its constituents and health; at present, there are 261 authorised health claims. We also collected information on the retail price of the products and their placement within each store.
Statistical analysis
Based on the list of ingredients of the label of each product, we calculated the percentage of GUMs that had added sugars. We also calculated the mean total sugar and free sugar content. When we calculated the mean free sugar content, we excluded products that did not report added sugars in the list of ingredients, and those that reported added sugars but had a total sugar content that was lower than the total sugar content of cow’s milk
To calculate the percentage of the total energy content of each product contributed by total sugars and free sugars, based on an estimate of 4 kcal per gram of sugar, we multiplied this amount by the number of grams of sugar contained in 100 ml of the product, and divided the result by the total kilocalories of energy. We applied the same approach to calculate the energy contribution of fats (9 kcal/g) and proteins (4 kcal/g).
Last of all, we calculated the number and percentage of products whose labelling or packaging included health or nutritional claims or endorsements from health or scientific institutions.
RESULTS
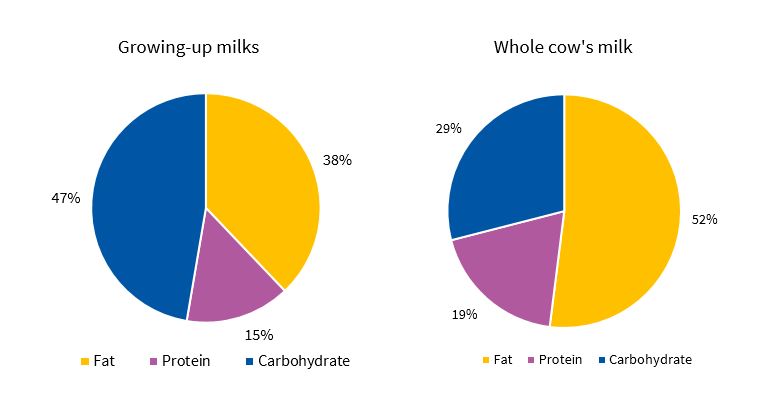
Of all GUMs, 70% (14/20) were made with low-fat milk and the rest with fat-free milk. The mean energy content of the GUMs under study was 67.7 kcal/100 ml, slightly above the energy content of whole cow’s milk (65 kcal/100 ml) and between 1.5 and 2 times the content of the low-fat and fat-free milks that these products are made with. The mean fat, protein and carbohydrate contents of the GUMs were 2.8, 2.5 and 8 g/100 ml, respectively, corresponding to 37.7%, 14.8% and 47.5% of the total energy content of the product. Thus, GUMs have a high sugar content and a low protein and fat content compared to whole cow’s milk, which is the food recommended for this age group (Fig. 1).
| Figure 1. Nutrient composition of whole cow’s milk and growing-up milks available in supermarkets in Badajoz (2017)) |
|---|
 |

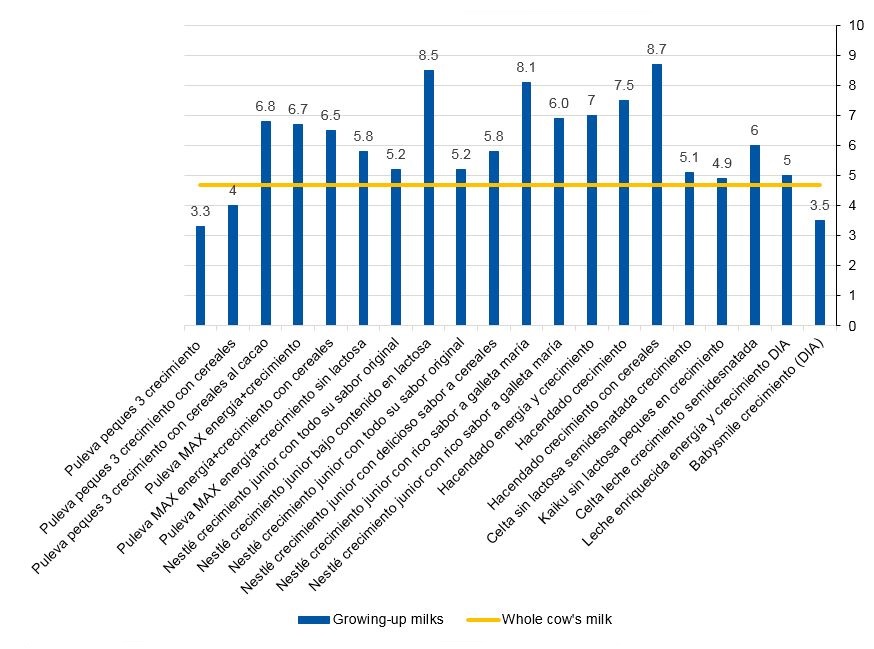
Of the 20 GUMs in the sample, 17 (85%) had added sugars in the ingredients list under one or more of the following denominations: sugar, fructose, sucrose, maltodextrin, honey and caramel (Table 1). The mean total sugar content was 6 g/100 ml of product, and the mean free sugar content was 2.1 g/100 ml. Of the 20 processed dairy products, 17 (85%) exceeded the total sugar content found in whole cow’s milk (4.7 g/100 ml), with values ranging between 4.9 and 8.7 g/100 ml (Fig. 2). However, three of these products did not declare the presence of added sugars in the ingredients list, with the most salient example being the Celta low-fat GUM, which contains 6 g of sugar per 100 ml (Table 1). We also identified 3 products that reported a total sugar content inferior to that of the low-fat milk they were made with, despite disclosing the presence of added sugars in the ingredients list (Table 1).
| Table 1. Sugar content of growing-up milks sold in supermarkets in Badajoz, 2017 | |||||
|---|---|---|---|---|---|
| Commercial product | Type of milk | Age | Added sugars * | Total sugar g/100 ml | Free sugar g/100 ml** |
| Puleva peques 3 crecimiento | Low-fat | +12 months | Sugar, maltodextrin, fructose | 3.3 | |
| Puleva peques 3 crecimiento con cereales | Low-fat | +12 months | Sucrose, maltodextrin, fructose | 4 | |
| Puleva peques 3 crecimiento con cereales al cacao | Low-fat | +12 months | Sucrose, maltodextrin, fructose | 6.8 | 2.2 |
| Puleva MAX energía + crecimiento | Low-fat | +3 years | Sugar, fructose | 6.7 | 2.1 |
| Puleva MAX energía + crecimiento con cereales | Low-fat | +3 years | Sugar, fructose | 6.5 | 1.9 |
| Puleva MAX energía + crecimiento sin lactosa | Low-fat | +3 years | Sugar | 5.8 | 1.2 |
| Nestlé crecimiento junior con todo su sabor original | Fat-free | +12 months | Dextrin-maltose, sugar, lactose | 5.2 | 0.6 |
| Nestlé crecimiento junior bajo contenido en lactosa | Fat-free | +12 months | Dextrin-maltose, sugar | 8.5 | 3.9 |
| Nestlé crecimiento junior con todo su sabor original | Fat-free | +2 years | Dextrin-maltose, sugar, lactose | 5.2 | 0.6 |
| Nestlé crecimiento junior con delicioso sabor a cereales | Fat-free | +12 months | Dextrin-maltose, sugar, lactose | 5.8 | 1.2 |
| Nestlé crecimiento junior con rico sabor a galleta maría | Fat-free | +12 months | Sugar, caramel | 8.1 | 3.5 |
| Nestlé crecimiento junior con rico sabor a galleta maría | Fat-free | +2 years | Sugar, caramel | 6.9 | 2.3 |
| Hacendado energía y crecimiento | Low-fat | +3 years | Sugar, honey | 7 | 2.4 |
| Hacendado crecimiento | Low-fat | +12 months | Sucrose, dextrin-maltose, honey | 7.5 | 2.9 |
| Hacendado crecimiento con cereales | Low-fat | +12 months | Sucrose, dextrin-maltose, honey | 8.7 | 4.1 |
| Celta sin lactosa semidesnatada crecimiento | Low-fat | +3 years | 5.1 | 0.5 | |
| Kaiku sin lactosa peques en crecimiento | Low-fat | +3 years | Fructose | 4.9 | 0.3 |
| Celta leche crecimiento semidesnatada | Low-fat | +3 years | 6 | 1.4 | |
| Leche enriquecida energía y crecimiento DIA | Low-fat | No age specified | 5 | 0.4 | |
| Babysmile crecimiento (DIA) | Low-fat | +12 months | Maltodextrin, sugar | 3.5 | |

The percentage of the total energy content in 100 ml of product contributed by the total sugars ranged between 20% and 48%, and the highest value corresponded to the product known as “Nestlé Crecimiento Junior bajo contenido en lactosa” (Nestlé Low-Lactose Junior Growth) (Table 2). Seventy percent of the products (14/20) exceeded the percent energy contributed by sugars naturally present in whole cow’s milk, which is 29%. As for free sugars, this percentage ranged between 3% and 22% of the total energy content. Of the 14 products for which we estimated the amount of free sugars, 50% exceeded the maximum amount recommended by the WHO (10% of the total energy) for this added nutrient.
| Table 2. Percentage of total energy contributed by total sugars and free sugars in growing-up milks available in Badajoz (2017) | |||||
|---|---|---|---|---|---|
| Commercial product | kcal/100 ml* | Total sugar (kcal/100 ml) | Percent of total energy contributed by total sugar** | Free sugar (kcal/100 ml) | Percentage of total energy contributed by free sugars** |
| Puleva peques 3 crecimiento | 60 | 13.2 | 22 | ||
| Puleva peques 3 crecimiento con cereales | 78 | 16 | 20 | ||
| Puleva peques 3 crecimiento con cereales al cacao | 90 | 27.2 | 30 | 8.8 | 10 |
| Puleva MAX energía + crecimiento | 62 | 26.8 | 43 | 8.4 | 13 |
| Puleva MAX energía + crecimiento con cereales | 77 | 26 | 34 | 7.6 | 10 |
| Puleva MAX energía + crecimiento sin lactosa | 59 | 23.2 | 39 | 4.8 | 8 |
| Nestlé crecimiento junior con todo su sabor original | 70 | 20.8 | 29 | 2.4 | 3 |
| Nestlé crecimiento junior bajo contenido en lactosa | 70 | 34 | 48 | 15.6 | 22 |
| Nestlé crecimiento junior con todo su sabor original | 71 | 20.8 | 29 | 2.4 | 3 |
| Nestlé crecimiento junior con delicioso sabor a cereales | 80 | 23.2 | 29 | 4.8 | 6 |
| Nestlé crecimiento junior con rico sabor a galleta maría (+ 12 months) | 83 | 32.4 | 39 | 14 | 17 |
| Nestlé crecimiento junior con rico sabor a galleta maría (+2 years) | 77 | 27.6 | 36 | 9.2 | 12 |
| Hacendado energía y crecimiento | 67 | 28 | 42 | 9.6 | 14 |
| Hacendado crecimiento | 65 | 30 | 46 | 11.6 | 18 |
| Hacendado crecimiento con cereales | 82 | 34.8 | 42 | 16.4 | 20 |
| Celta sin lactosa semidesnatada crecimiento | 48 | 20.4 | 42 | 2 | 4 |
| Kaiku sin lactosa peques en crecimiento | 46 | 19.6 | 43 | 1.2 | 3 |
| Celta leche crecimiento semidesnatada | 60 | 24 | 40 | 5.6 | 10 |
| Leche enriquecida energía y crecimiento DIA | 47 | 20 | 42 | 1.6 | 3 |
| Babysmile crecimiento (DIA) | 61 | 14 | 23 | ||

All the GUMs under study included nutritional claims, with expressions along the lines of “source of…” or “enriched with…” in reference to the presence of omega-3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), various vitamins and minerals such as calcium, zinc, iron, iodine, magnesium and phosphorus. Sixty-percent of the products (12/20) displayed health claims that have not been authorised by the European Food Safety Authority (EFSA). Specifically, the six products by Puleva® bore the following unauthorised claim: “It is a source of iron, contributes to adequate learning in children” as opposed to “iron contributes to normal cognitive development of children”. The three “Puleva Max” products bore an additional unauthorised claim: “Replaces part of the saturated fat with unsaturated fat, such as omega-3 DHA present in breast milk. It is a component of retinal cells”. The four “Nestlé crecimiento junior” varieties for children aged more than 1 year bore the following unauthorised claim: “Zinc: helps growth”. The two “Hacendado crecimiento” products targeting children aged more than 1 year claimed having a “Fat, protein and carbohydrate contents modified according to nutrition guidelines for children”, which does not fit any of the nutritional claims authorised by the European Commission regulation.
In the 12 (60%) products distributed under the Nestlé® and Puleva® brands, the front of the package displayed the following statement: “Nestlé/Puleva collaborates with the Asociación Española de Pediatría”, in the case of Puleva® displaying the initials of this association (AEP) in a larger font compared to the rest of the sentence, and in the case of Nestlé®, with a larger font and with the word “PEDIATRÍA” printed in capital letters.
The prices of the products included in the study ranged between 0.92 and 1.67 € per litre, that is, between twice and three times the price of whole cow’s milk. When it came to their placement in supermarkets, GUMs that targeted children aged 3 or more years were displayed in the aisle devoted to dairy products, while GUMs targeted to children aged 1 and 2 years were in the aisle devoted to infancy and early childhood nutrition.
DISCUSSION
In 2017, the GUMs for children aged 1 to 3 years available in supermarkets in Badajoz had high energy and sugar contents and low protein and fat contents compared to whole cow’s milk. Eighty-five percent of analysed GUMs had added sugars in their ingredient list, which goes against the recommendations of the WHO, and in some instances the total sugar content was twice that of in whole cow’s milk. Free sugars accounted for 3% to 22% of the total energy content of these GUMs. All GUMs bore some type of nutritional or health claim. Twelve products (60%) bore unauthorised health claims, and the same number had the endorsement of the AEP.
The market of food products for young children is divided between a small number of manufacturers, and those based in the European Union are world leaders in the sector.18 Thus, it is not surprising that previous studies that have analysed the nutrient composition of GUMs and similar products for young children had results similar to ours in terms of nutrient composition and sugar content.19,20 The detection of three GUMs that had a lower total sugar content compared to cow’s milk despite the disclosure of added sugars in their list of ingredients suggests that the percentage of milk in some of these products may be very low, to the point that milk may not be the main ingredient in some of them despite their presentation as a GUM. In fact, the main ingredient in one of the products with the highest contents of sugar was water. Furthermore, the fact that three of the products had a higher total sugar content compared to the low-fat milk with which they were made while their ingredients lists did not include added sugars evinces errors or omissions in the disclosure of ingredients, especially in the case of the “Celta leche crecimiento semidesnatada” (Celta low-fat grow-up milk); in another 2 cases, the difference in relation to cow’s milk was so small that it could be due to chance variations in measurement. In addition to the potential presence of such errors, consumers are unable to know the amount of free sugars present in these foods by reading nutrition facts tables only, as these do not discriminate between added sugars and those naturally present in the food, which makes it necessary to also consult the list of ingredients to see whether the product contains added sugars or not. Since added sugars are the sugars associated with obesity, dental caries and cardiovascular risk factors, it is essential that action be taken in regard to the nutrient composition of these foods and to regulate their presentation and marketing. Imposing the use of interpretive nutrition labels, more easily understood by consumers, and the explicit differentiation between the content of sugars naturally present in the food versus added or free sugars released during the manufacturing process would be sensible measures to take.
All the GUMs in this study bore nutritional claims about their vitamin and mineral contents, the daily requirements of which can be easily met by keeping a balanced diet without need for enrichment. Thus, the latter is unnecessary, and it may even have a negative impact on child health, for instance if calcium or vitamin D are consumed in excessive amounts.21 21 Furthermore, a recent study demonstrated that children that consume whole cow’s milk gain less weight and have higher levels of vitamin D compared to children that consume fat-free milk.22 On the other hand, consumption of GUMs, which is very common between ages 12 and 18 months, has been associated with an increased risk of childhood obesity.23
The display of health claims that are unauthorised by the EFSA is a form of false advertising. This type of fraud is highly relevant, as the main motivation reported by most consumers for reading food labels is choosing healthier products.24 In addition, these claims make it possible to increase the price of the product, in some cases nearly tripling the price of whole cow’s milk, taking advantage of the fact that consumers are willing to pay more for products bearing claims that imbue them with a healthy aura.25 25 While the law allows the display of endorsements from the AEP, this can also constitute false advertising and is questionable from a professional ethics standpoint when it is given, as is the particular case that we are analysing in this article, to products that do not adhere to the recommendations of the WHO and nutrition experts.7,8,13,14 Consumers, who are defenceless in the face of health claims unsupported by scientific evidence and the spurious use of nutritional claims and endorsements from associations of health professionals, are misled by the healthy mystique that surrounds GUMs and may purchase them despite them being more expensive and less suitable to their children than whole cow’s milk.
In 2013, the EFSA developed a technical report at the behest of the European Commission that concluded that GUMs are a possible means to increase intake of polyunsaturated omega-3 fatty acids, , iron and vitamin D in infants and young children, although noting that there are effective alternatives for increasing the intake of these nutrients.26,27 Finally, in the scientific opinion issued on June 26, 2014, the EFSA noted that young children can continue taking the infant and follow-up formulae consumed during the first year of life, and thus did not consider it necessary to establish a specific composition for young-child formulae.27 However, an external report commissioned by the EFSA evinced the widespread presence of GUMs in the markets of all European Union members, with an increasing trend in consumption in nearly all. The countries with the highest consumption and the widest variety of available products were France, Spain, Italy and Germany.18 The high sugar content of these products, which contravenes the recommendations of the WHO and nutrition experts, underscores the need to establish specific regulations regarding their composition28, in order to protect the paediatric population during early childhood, a period where individuals are particularly vulnerable. Furthermore, the preamble of the Regulation (EC) on Nutrition and Health Claims announced the future application of nutrient profiles to ensure that only foods and beverages with a healthy nutrient profile can bear health claims. In the case of GUMs, this would entail the prohibition of using claims or elements implying the support of scientific or health institutions or groups in nearly all products currently available in the market due to the presence of added sugars.
One possible limitation of our study is its small sample size, as we only obtained products from supermarkets in the city of Badajoz. However, the supermarket chains we visited included most of the chains with a nationwide distribution, and the products available for retail are manufactured by a small number of multinational food companies, so that the supply does not vary significantly between supermarkets, so we do not think that the size and composition of the sample would have varied significantly had we included other cities in the sampling. Another limitation is the lack of information on added sugars in the nutrition facts label, due to which we had to estimate the amount of free sugars in GUMs. Since we made this estimation by calculating the difference in their total sugar contents compared to cow’s milk and we did not know the percentage of milk contained in each product, we may have underestimated the amount of free sugars in some cases.
To conclude, we believe it necessary to regulate GUMs, whose consumption by young children is increasing, due to their high added sugar content, which contravenes the recommendations of the WHO and experts in nutrition. The regulation should address the nutrient composition of the products, demand more detailed labelling with an interpretive format and prohibit the use of nutritional or health claims and the backing of scientific or health institutions in products targeted to young children that do not adhere to current nutritional guidelines, especially products containing added sugars.
NOTE FROM THE AUTHORS
This article presents independent research and findings. The opinions expressed in the article are those of the authors and do not necessarily represent the official position of the Instituto de Salud Carlos III.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article.
ABBREVIATIONS
AEP: Asociación Española de Pediatría; BEDCA: Base de Datos Española de Composición de Alimentos; DHA: docosahexaenoic acid; EFSA: European Food Safety Authority; EPA: eicosapentaenoic acid; GUM: growing-up milk ; WHO: World Health Organization.
REFERENCES
- Sánchez Cruz JJ, Jiménez Moleón JJ, Fernández Quesada F, Sánchez MJ. Prevalence of child and youth obesity in Spain in 2012. Rev Esp Cardiol (Engl Ed). 2013;66:371-6.
- Ramiro González MD, Sanz Barbero B, Royo Bordonada MA. Childhood excess weight in Spain from 2006 to 2012. Determinants and parental misperception. Rev Esp Cardiol. 2017;70:656-63.
- Monasta L, Batty GD, Cattaneo A, Lutje V, Ronfani L, Van Lenthe FJ, et al. Early-life determinants of overweight and obesity: a review of systematic reviews. Obes Rev. 2010;11:695-708.
- Vos M, Kaar J, Welsh J, Van Horn L, Feig D, Anderson C, et al. Added sugars and cardiovascular disease risk in children: a scientific statement from the American Heart Association. Circulation. 2016;135:e1017-e1034.
- Drewnowski A, Mennella JA, Johnson SL, Bellisle F. Sweetness and food preference. J Nutr. 2012;142:1142S-1148S.
- Tryon M, Stanhope K, Epel E, Mason A, Brown R, Medici V, et al. Excessive sugar consumption may be a difficult habit to break: a view from the brain and body. J Clin Endocrinol Metabol. 2015;100:2239-47.
- Informe del Consejo Ejecutivo sobre su 106.ª y 107.ª reuniones de la OMS. A54/2. 30 de marzo de 2001. En: Organización Mundial de la Salud [en línea] [consultado el 06/11/2018]. Disponible en http://apps.who.int/gb/archive/pdf_files/WHA54/sa542.pdf
- Conclusiones del Consejo para contribuir a detener el aumento de sobrepeso y la obesidad infantil 2017/C205:46-52. En: Diario Oficial de la Unión Europea [en línea] [consultado el 06/11/2018]. Disponible en http://eur-lex.europa.eu/legal-content/ES/TXT/PDF/?uri=CELEX:52017XG0629(01)&from=ES
- Resolución WHA63.14 sobre la promoción de alimentos y bebidas no alcohólicas dirigida a los niños, aprobado por la 63.ª Asamblea Mundial de la Salud. En: Organización Mundial de la Salud [en línea] [consultado el 06/11/2018]. Disponible en http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_R14-sp.pdf
- Moynihan P, Kelly S. Effect on caries of restricting sugars intake. J Dental Res. 2013;93:8-18.
- Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2012;346:e7492-e7492.
- Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98:1084-102.
- Galiano Segovia MJ, Moreno Villares JM. La leche de vaca en la alimentación del niño: ¿necesaria o causa de problemas? Pediatr Integral. 2013;17:371-6.
- Dalmau J. Nutrición en la infancia y en la adolescencia. En: Carbajal A, Martínez C (coords.). Manual práctico de nutrición y salud. Madrid: Exlibris Ediciones; 2012. p. 207-21.
- Informe de la Comisión al Parlamento Europeo y al Consejo sobre los preparados para niños de corta edad. Bruselas: 31.3.2016. COM (2016) 169 final. En: Comisión Europea [en línea] [consultado el 06/11/2018]. Disponible en https://ec.europa.eu/transparency/regdoc/rep/1/2016/ES/1-2016-169-ES-F1-1.PDF
- Sugars intake for adults and children. En: Organización Mundial de la Salud [en línea] [consultado el 06/11/2018]. Disponible en who.int/nutrition/publications/guidelines/sugars_intake/en/
- Basulto J, Ojuelos FJ, Baladia E, Manera M. Azúcares en alimentos infantiles. La normativa española y europea, ¿a quién protege? Rev Pediatr Aten Primaria. 2016;18:e47-e53.
- AINIA Centro Tecnológico. Report of data collection with respect to the availability and nutritional composition of different types of milk-based drinks and similar products for young children with the denomination of “growing up milks” or “toddlers milk” or with similar terminology currently on the market in EU member States. EFSA supporting publication 2013: EN-505. En: EFSA Online Library [en línea] [consultado el 06/11/2018]. Disponible en http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2013.EN-505/epdf
- La alimentación industrializada del lactante y el niño pequeño. El nuevo meganegocio. El poder de consumidor. En: Ministerio de Salud. Gobierno de Costa Rica [en línea] [consultado el 06/11/2018]. Disponible en ministeriodesalud.go.cr/gestores_en_salud/lactancia/articulos/CNLM_alimentacion_industrializada_lactante_nino_pequeno.pdf
- Jardí C, Aranda N, Bedmar C, Arija V. Composición nutricional de las leches infantiles. Nivel de cumplimiento en su fabricación y adecuación a las necesidades nutricionales. An Pediatr (Barc). 2015;83:417-29.
- Molina H, Mena P, Vial P, Fernández ME, Alcázar ML, Muzzo S. Intoxicación por vitamina D en el lactante. Rev Chil Pediatr. 1984;55:270-3.
- Morency M, Birken C, Lebovic G, Chen Y, L’Abbé M, Lee G, et al. Association between noncow milk beverage consumption and childhood height. Am J Clin Nutr. 2017;106:597-602.
- Wiberger M, Eiben G, Lissner L, Mehlig K, Papoutsou S, Hunsberger M. Children consuming milk cereal drink are at increased risk for overweight: the IDEFICS Sweden study, on behalf of the IDEFICS Consortium. Scan J Public Health. 2014;42:518-24.
- Prieto Castillo L, Royo Bordonada MA, Moya Geromini A. Information search behaviour, understanding and use of nutrition labeling by residents of Madrid, Spain. Public Health. 2015;129:226-36.
- De Magistris T, López Galán B. Consumers’ willingness to pay for nutritional claims fighting the obesity epidemic: the case of reduced-fat and low salt cheese in Spain. Public Health. 2016;135:83-90.
- Panel on Dietetic Products, Nutrición and Allergies (NDA). Scientific opinion on nutrient requirements and dietary intakes of infant and young children in the European Union. EFSA J. 2013;11:3408.
- EFSA Panel on Dietetic Products, Nutrición and Allergies (NDA). Scientific opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014;12:3760.
- Palou Óliver, A, Palou March M. La evidencia científica la información al consumidor: las declaraciones nutricionales y de propiedades saludables (health claims) en los alimentos. Rev Esp Comun Salud. 2016;S1:31-42.