Vol. 20 - Num. 77
Original Papers
Association of dairy consumption with respiratory infections. Myth or reality?
Diego Mauricio Peñafiel Freirea, Nerea Martín Calvob, Lorena García Blancoc, Itziar Zazped, Noelia Álvarez Zalloe, Laura Moreno Galarragaf
aServicio de Pediatría. Complejo Hospitalario de Navarra. Pamplona. España.
bDepartamento de Medicina Preventiva y Salud Pública. Universidad de Navarra. Pamplona. España.
cPediatra. CS Ansoáin. Ansoáin. Navarra. España.
dDepartamento de Ciencias de la Alimentación y Fisiología. Universidad de Navarra. Pamplona. España.
eServicio de Urgencias Extrahospitalarias. Servicio Navarro de Salud-Osasunbidea. Pamplona. España.
fServicio de Pediatría. Complejo Hospitalario de Navarra. Servicio Navarro de Salud. Pamplona. Instituto de Investigación Sanitaria de Navarra (IdiSNA). Pamplona. España.
Correspondence: DM Peñafiel. E-mail: dpfreire.89@gmail.com
Reference of this article: Peñafiel Freire DM, Martín Calvo N, García Blanco L, Zazpe I, Álvarez Zallo N, Moreno Galarraga L. Association of dairy consumption with respiratory infections. Myth or reality? Rev Pediatr Aten Primaria. 2018;20:45-52.
Published in Internet: 15-02-2018 - Visits: 50616
Abstract
Introduction: milk and dairy products are important nutrients in child development. However, the belief that they are associated with respiratory infections is leading to restrictions in their consumption or their replacement by plant-based milks. The objective of our study was to analyse the potential association between dairy consumption and certain respiratory infections in children.
Materials and methods: we conducted a cross-sectional study in 169 children aged 4 to 7 years that participated on a voluntary basis. The data were collected through paper-based questionnaires. We collected data on dietary habits through a 151-item semiqualitative food frequency questionnaire. We assessed the association of the consumption of milk, cheese and yoghurt with specific respiratory diseases (acute otitis media, sinusitis, mastoiditis, pneumonia), comparing two groups of participants defined by their consumption relative to the median for each food, and using multivariate logistic regression.
Results: we found no association between the consumption of dairy products and the respiratory diseases under study (odds ratio: 0.85; 95% confidence interval: 0.44 to 1.64]). In the separate analysis of each dairy category, we found an inverse correlation between consumption of cheese and overall respiratory disease (odds ratio: 0.50; 95% confidence interval: 0.26 to 0.98), but no association with a specific infection (acute otitis media or pneumonia). We found no significant differences in outcomes for any other dairy category (milk or yoghurt).
Conclusions: our results did not find a direct association between dairy consumption and respiratory infections in children. The current data do not support restricting consumption of milk or dairy products in school-aged children.
Keywords
● Dairy products ● Otitis media ● Pneumonia ● Respiratory tract infectionsINTRODUCTION
There is a widespread belief in the community that consumption of dairy products is associated with the development of various childhood respiratory illnesses.1,2 More specifically, it is believed that consumption of cow’s milk protein (CMP) is associated with an increase in mucus secretion in the respiratory tract and in respiratory infection. Although the current evidence does not warrant it,3 in actual clinical practice parents of children with respiratory diseases often restrict their consumption of CMP or replace it by plant proteins or lactose-free, soy or rice milks and products.
Dairy products constitute an essential food group in the physical development of infants and children,4 and their consumption has been associated with improved academic performance.5 Furthermore, the calcium content of dairy products contributes to the adequate development of bone mass and the control of blood pressure.6-8 The recommended dairy intake for school-aged children is of 2-4 servings a day.9
Several cross-sectional studies have even suggested that consumption of dairy may actually protect against respiratory disease. A study conducted in New Zealand found that consumption of milk and eggs in the past 12 months was associated with a significant decrease in the incidence of wheezing.10 Along the same lines, other authors have described an increased incidence of respiratory symptoms, especially those associated with bronchitis and asthma, in individuals with a lower intake of milk and dairy products.11,12 As for mucus production, a previous study did not find any difference between cow’s milk and soy placebo.13
Notwithstanding, due to social pressure, the increased availability of alternatives to milk and dairy products and the current lack of scientific evidence, many parents are choosing to restrict dairy consumption in their children or substituting soy- or rice-based products. Thus, the aim of our study was to analyse the association of the consumption of cow’s milk and dairy products (cheese and yoghurt) with a series of childhood respiratory diseases.
MATERIALS AND METHODS
Study design
We conducted a cross-sectional study that constituted the pilot of the project Seguimiento de Escolares Navarros para un Desarrollo Óptimo (Followup of School Children in Navarre for Optimal Development [SENDO]), a prospective, multi-purpose study in a paediatric cohort to analyse the impact of diet and lifestyle on the development of different diseases in children and adolescents. More information on the SENDO project can be found in its website (www.proyectosendo.es).
Sample selection
We recruited participants for the study through the primary care paediatricians of the Public Health System of Navarre (Servicio Navarro de Salud-Osasunbidea [SNS-O]) between February and April 2015. Participation was voluntary, and the parents or legal guardians of every participant signed an informed consent form. The inclusion criteria were residence in the autonomous community of Navarre (located in northern Spain) and having been born between January 2008 and December 2010. There were no exclusion criteria.
The initial sample included 170 children for who the research team had received completed baseline questionnaires by September 2016. We excluded one of these children due to a reported energy intake outside the established bounds (between 550 and 3800 kcal/day),14 so the final sample consisted of 169 children.
Data collection
Data on exposure
We collected data through paper questionnaires completed by the parents of the participants. We collected information on sociodemographic characteristics, lifestyle and family and personal history.
The data on dairy consumption was collected in a semiquantitative food frequency questionnaire (FFQ) that included 151 foods divided into 10 categories (dairy; eggs, meat and fish; vegetables; fruits; legumes and cereals; oils and fats; candies and snacks; drinks; baked goods; others). Each participant had to report their average consumption of each of the foods during the previous year on a 9-point scale that ranged from never or nearly never to more than 6 times a day.
We analysed the effect of consuming milk, cheese and yoghurt separately and in combination, defining the variable “dairy” as the combination of all three. We measured consumption in servings/day. The questionnaires specified that a serving of milk corresponded to 200 ml, and servings of cheese and yoghurt to 30 g and 125 g, respectively.
The “milk” variable included the consumption of whole, low-fat and fat-free milk, follow-on formulas, calcium-enriched milk, vitamin-enriched milk and milk shakes. We did not include lactose-free milk or plant-based milks. The “cheese” variable included consumption of sliced cheese, pre-packaged snack-sized cheeses, white cheeses and other. Lastly, the “yoghurt” variable included consumption of any type of yoghurt, including flavoured yoghurt, yoghurt with fruit chunks, cuajada, custard, dairy products based on fresh cheese and fermented drinks containing L. casei inmunitas.
To assess the impact of the consumption of milk, yoghurt, cheese and dairy overall, participants were classified into two groups based on whether their own consumption was above or below the median consumption (dairy overall, 4 servings/day; milk, 2.5 servings/day; cheese, 0.40 servings/day and yoghurt, 1.30 servings/day). We used the lower-consumption group as the reference.
Data on outcomes
In the paper-based questionnaires, we asked whether the participant had received a diagnosis by a physician of any of several diseases, including acute otitis media (AOM), sinusitis, mastoiditis and pneumonia. We specifically asked about these diseases because they have a common pathophysiological mechanism that includes an increase in mucus secretion in the airways and adjacent cavities. We chose to study them because one of the negative effects attributed to dairy consumption is an increased production of mucus and respiratory secretions in children, with an associated increase in ear and respiratory tract infections. Despite their high prevalence, we did not include upper respiratory tract infections (URTIs) because many such episodes are mild and are not managed by a health professional, which makes it difficult to determine the actual number of URTI episodes experienced by a school-aged child and therefore to obtain valid data. We also excluded children with underlying diseases that could manifest with increased respiratory secretions, such as cystic fibrosis, primary ciliary dyskinesia, bronchiectasis, allergies or immunodeficiency disorders.
Other variables under study
We collected data on the weight, height and waist circumference of participants. We calculated the body mass index (BMI) dividing the weight (kg) by the square of the height (m2), and the waist-to-height ratio (WHtR) dividing the waist circumference (cm) by the height (cm). Both the BMI and the WHtR correlate strongly to adiposity.15 We also collected data on breastfeeding. We used single imputation to replace missing data.
Statistical analysis
We compared quantitative variables with the Student t test, and proportions by means of the χ2 test or the Fisher exact test as applicable.
To analyse the association between dairy consumption and the infections under study, we performed multivariate logistic regression adjusted for sex, age, BMI, breastfeeding history and total energy intake. We used the low-consumption category as the reference in all the analyses. To fine-tune the adjustment for total energy intake, we performed an additional analysis where we adjusted dairy consumption for the total energy intake using the residual method.
We performed the statistical analysis with the STATA 12.0®software. All the tests were two-tailed, and statistical significance was defined as a p-value of less than 0.05.
Ethical considerations
The parents or legal guardians of every participant signed an informed consent form. The study was approved by the Clinical Research Ethics Committee of Navarre and by the Research Ethics Committee of the Universidad de Navarra (Spain).
RESULTS
The final sample included a total of 169 children (56.2% female) born between 2008 and 2010 (mean age, 6.1 years; standard deviation [SD], 0.92) (Table 1). The mean BMI was 15.77 (SD: 1.71) and the mean WHtR was 0.47 (SD: 0.05). The total energy intake was significantly higher and the WHtR significantly lower in the high dairy consumption group compared to the low dairy consumption group. We did not find any other significant differences in the rest of the variables under study.
| Table 1. Sociodemographic and anthropometric characteristics of participants by overall dairy consumption | ||||
|---|---|---|---|---|
| Low dairy consumption: ≤4 servings/day (n = 85) | High dairy consumption: >4 servings/day (n = 84) | Total (n = 169) | P | |
| Age (years) | 6 (0.9) | 6.1 (0.9) | 6.1 (0.9) | .31 |
| Sex (female) | 53 (62.3%) | 42 (50%) | 95 (56.2%) | .11 |
| Weight (kg) | 21.6 (4) | 22 (3.4) | 21.8 (3.7) | .51 |
| BMI (kg/m2) | 15.9 (1.9) | 15.6 (1.3) | 15.8 (1.6) | .17 |
| BMI z-score | - 0.1 (0.9) | - 0.2 (0.7) | - 0.1 (0.8) | .38 |
| Waist to height ratio (WHtR) | 0.48 (0.01) | 0.46 (0.01) | 0.47 (0.004) | .03 |
| Energy intake (kcal/day) | 1690.3 (414.7) | 2011.2 (405.6) | 1849.8 (439.5) | <.01 |
| Breastfeeding | 72 (85.7%) | 68 (78.3%) | 137 (82%) | .21 |
| Number of siblings | 2.7 (1.9) | 3.1 (1.8) | 2.9 (1.8) | .14 |

Our study found a mean dairy consumption of 4.11 servings/day (range, 0.29-9.93 servings/day). The mean consumption of milk was 2 servings/day (range, 0-6.20), the mean consumption of cheese 0.62 servings/day (range, 0-5.43 servings/day) and the mean consumption of yoghurt 1.49 servings/day (range, 0-8.57 servings/day). Twelve children reported not consuming cow’s milk or derived products at all.
As for the outcomes, 58 children in the sample (34.3%) reported having received a diagnosis by a physician of at least one of the diseases of interest, specifically AOM (25.4%), sinusitis (1.2%) and pneumonia (12.4%).
Although point estimates in the crude analysis and the adjusted models suggested a potential inverse correlation, we did not find a significant association between overall dairy consumption and the overall incidence of the diseases under study (Table 2).
| Table 2. Odds ratio (95 CI) for the association of the consumption of dairy and respiratory diseases | ||
|---|---|---|
| Low dairy consumption: ≤4 servings/day (n = 85) |
High dairy consumption: >4 servings/day (n = 84) |
|
| Crude model | 1.00 (Ref.) | 0.92 (0.49 to 1.73) |
| Model adjusted for sex and age | 1.00 (Ref.) | 0.85 (0.44 to 1.63) |
| Multivariate model* | 1.00 (Ref.) | 0.78 (0.39 to 1.59) |
| Multivariate model adjusted by means of residual method | 1.00 (Ref.) | 0.85 (0.44 to 1.64) |

The adjusted model showed a 15% decrease in the relative risk in the high dairy consumption group (>4 servings/day), although the differences between groups were not statistically significant. The separate analyses for each of the three types of dairy under study (milk, yoghurt and cheese) (Figure 1) showed that a higher consumption of cheese was associated with a significantly lower overall risk of disease (odds ratio [OR]: 0.50; 95% confidence interval [95 CI]: 0,26 a 0,98]). However, we found no significant association with milk (OR: 1.02; 95 CI: 0.53 to 1.97) or yoghurt (OR: 0.91; 95 CI: 0.47 to 1.75).
| Figure 1. Odds ratio and 95 CI of the overall risk of respiratory infection and the risk of pneumonia and otitis media in particular based on the consumption of the three types of dairy (high consumption versus low consumption) |
|---|
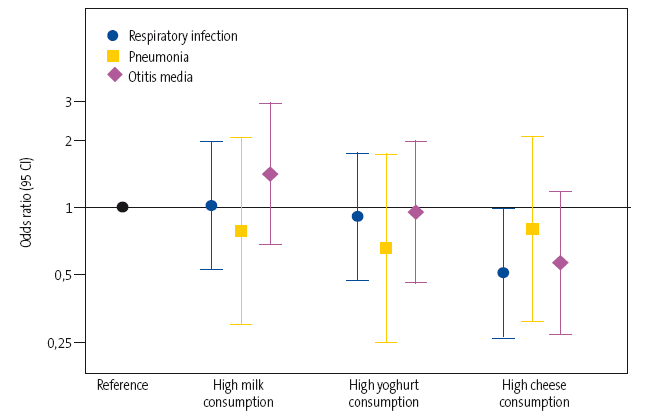 |

Similarly, although in the separate analyses for each of the diseases under study we found estimates that suggested an inverse correlation between the consumption of cheese or of yoghurt and the development of pneumonia (OR: 0.66 [95 CI: 0.25 to 1.73] and OR: 0.81 [95 CI: 0.31 to 2.07], respectively) and of AOM (OR: 0.95 [95 CI: 0.46 to 1.96] and OR: 0.57 [95 CI: 0.27 to 1.17], respectively), we found no statistically significant associations in the multivariate analysis.
DISCUSSION
We conducted a cross-sectional study in 169 children to analyse whether a greater consumption of dairy was associated with an increased risk of certain childhood infectious diseases (AOM, sinusitis and pneumonia), and found results that were not statistically significant and did not support the existence of such an association.
Children in our sample reported a mean consumption of 4.11 servings/day of dairy (milk and milk products), which is at the upper limit of the recommended intake for this age group. The mean consumption of milk was 2 servings/day, which corresponded to 400 ml, and also reflected a greater intake than recommended for children that age.9 We ought to note that 12 participants (7%) reported that they did not consume cow’s milk at all, a food that has been proven to be important in the diet of school-aged children.4,16
We found a higher total energy intake in the group that reported a greater consumption of dairy (milk and milk products). However, the rest of the variables, including anthropometric measurements, were not significantly different between the two groups. The BMI z‑scores of the children in the sample (z = -0.1) were appropriate for their age and sex.
The lack of results suggesting an association between overall dairy consumption and the infections under study was consistent with other studies.2,12,13,17 In the separate analysis of each of the types of dairy we found particular OR values suggesting a potential inverse correlation for yoghurt and cheese. Specifically, the risk of respiratory infection was reduced by 50% (95 CI: 2 to 74) in the group that reported a higher consumption of cheese (> 0.42 servings/day). Taking into account the sample size and the lack of scientific evidence on this association, we must interpret these findings with caution. New studies with larger sample sizes and an appropriate design are required before we can conclude that there is an actual physiological association between the consumption of cheese and the development of respiratory infections.
The broad variability in dairy consumption and the comprehensive data collection are two of the strengths of our study. However, we must mention some of its limitations. Participation in the SENDO project is voluntary and therefore the sample is not representative: neither the data on dairy consumption nor the prevalence of the diseases under study can be generalized to the population. However, the design of the study does allow the assessment of the association between the consumption of certain foods and the development of specific diseases. Most participants in our sample were Caucasian and from families with a high socio-cultural status (> 65% of parents had undergraduate or graduate degrees). Although further and better studies are needed to replicate our results in more diverse populations, the pathophysiological mechanisms that would account for the potential associations under study are not likely to be specific to a particular ethnicity or socioeconomic status. The most important concerns in cohort studies are the validity of self-reported data and the commitment of participants throughout the follow-up period. There have been important cohort studies that had samples from very specific groups that were not representative of the general population, such as nurses (the Nurses’ Health Study in Boston) or university graduates (SUN study in Navarre).18
While the diseases under study are very different in terms of their aetiology and epidemiology, we decided to analyse them as a group because all of them manifest with increased mucus secretion, which is the factor that has been hypothesised to be associated with dairy consumption, and while we found a considerable number of respiratory infections (34%), our study may have lacked statistical power due to its small sample size. Furthermore, although the use of self-reported data has been validated in multiple studies in the paediatric population,19,20 their use carries a risk of differential information bias, with a tendency to bias the measure of association toward the null. Last of all, the observational design of the study did not allow us to fully rule out the possibility of confounding due to variables that were either not taken into account or not controlled properly.
In conclusion, our study did not find an association of increased dairy consumption with an increased incidence of infection, which we analysed separately and overall (AOM and pneumonia). Our results do not support the elimination or replacement of cow’s milk or daily products in the diet of children with the aim of reducing the incidence of ear and respiratory tract infections. Cow’s milk continues to be an important food in the nutrition of school-aged children.
Further studies with an appropriate design and a long duration of followup are required to replicate our results.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article.
Ignacio H. De Larramendi research grant from the Fundación MAPFRE 2015 (grant of 15 000 euro for the development of the SENDO 2015 pilot study and initiation of the SENDO cohort study).
Young Researchers 2006 grant from the Sociedad Española de Neumología Pediátrica (grant of 5000 euro for research in the framework of the SENDO project on the impact of dairy consumption in school-aged children).
ABBREVIATIONS
AOM: acute otitis media • BMI: body mass index • CMP: cow’s milk protein • FFQ: food frequency questionnaire • OR: odds ratio • SD: standard deviation • SENDO: Seguimiento de Escolares Navarros para un Desarrollo Óptimo • SNS-O: Servicio Navarro de Salud-Osasunbidea • URTI: upper respiratory tract infection • WHtR: waist to height ratio.
ACKNOWLEDGMENTS
We thank the MAPFRE Foundation and the Sociedad Española de Neumología Infantil (Spanish Society of Paediatric Pulmonology). The SENDO project has been carried out thanks to the collaborative efforts of the Universidad de Navarra and the Primary Care system of the public health system of Navarre (Servicio Navarro de Salud-Osasunbidea). Therefore, we want to express our gratitude for the collaboration of all the primary care paediatricians of the public health system of Navarre and the Complejo Hospitalario de Navarra and the researchers of the Universidad de Navarra. Lastly, we thank all participants and their families for their willingness to collaborate with the SENDO project.
REFERENCES
- Woods RK, Wiener JM, Abramson M, Thien F, Walters EH. Patient’s perceptions of food induced asthma. Aust N Z J Med. 1996;26:504-12.
- Lee C, Dozor AJ. Do you believe milk makes mucus? Arch Pediatr Adolesc Med. 2004;158:601-3.
- Thiara G, Goldman RD. Milk consumption and mucus production in children with asthma. Can Fam Physician. 2012;58:165-6.
- Navas López VM, Sierra Salinas C. Errores y mitos en la alimentación infantil. In: Rivero Urgell M, Moreno Aznar LA, Dalmau Serra J. Libro blanco de la nutrición infantil en España. Zaragoza: Prensas de la Universidad de Zaragoza; 2015. p. 131-7.
- MacLellan D, Taylor J, Wood K. Food intake and academic performance among adolescents. Can J Diet Pract Res. 2008;69:141-4.
- Lanou AJ, Berkow SE, Barnard ND. Calcium, dietary products, and bone health in children and young adults: a re-evaluation of the evidence. Pediatrics. 2005;115:736-43.
- Huncharek M, Muscat J, Kupelnick B. Impact of dairy products and dietary calcium on bone-mineral content in children: results of meta-analysis. Bone. 2008;43:312-21.
- Reid IR, Ames R, Mason B, Bolland MJ, Bacon CJ, Reid HE, et al. Effects of calcium supplementation on lipids, blood pressure, and body composition in healthy older men: a randomized controlled trial. Am J Clin Nutr. 2010;91:131-9.
- Moreiras O, Carbajal A, Cabrera L, Cuadrado C. Tablas de composición de alimentos. Guía de prácticas. Madrid: Ediciones Pirámide; 2016.
- Mitchell EA, Stewart AW, Clayton T, Asher MI, Ellwood P, Mackay R, et al. Cross-sectional survey of risk factors for asthma in 6-7-year-old children in New Zealand: International Study of Asthma and Allergy in Childhood Phase Three. J Paediatr Child Health. 2009;45:375-83.
- Lumia M, Takkinen HM, Luukkainen P, Kaila M, Lehtinen-Jacks S, Nwaru B, et al. Food consumption and risk of childhood asthma. Pediatr Allergy Immunol. 2015;26:789-96.
- Arney WK, Pinnock CB. The milk mucus belief: sensations associated with the belief and characteristics of believers. Appetite. 1993;20:53-60.
- Pinnock CB, Arney WK. The milk-mucus belief: sensory analysis comparing cow’s milk and a soy placebo. Appetite. 1993;20:61-70.
- Ortega RM, Navia B, López-Sobaler AM, Aparicio A. Ingestas diarias recomendadas de energía y nutrientes para la población española. Madrid: Universidad Complutense de Madrid; 2014.
- Martín Calvo N, Moreno Galarraga L, Martínez González MA. Association between body mass index, waist-to-height ratio and adiposity in children: a systematic review and meta-analysis. Nutrients. 2016;8:512.
- Pastor Martín MR. Programación de menús infantiles. In: Rivero Urgell M, Moreno Aznar L A, Dalmau Serra J. Libro blanco de la nutrición infantil en España. Zaragoza: Prensas de la Universidad de Zaragoza; 2015. p. 325-31.
- Wüthrich B, Schmid A, Walther B, Sieber R. Milk consumption does not lead to mucus production or occurrence of asthma. J Am Coll Nutr. 2005;24:547-55.
- Bes Rastrollo M, Martínez González MA. Ventajas y limitaciones de los grandes estudios epidemiológicos de seguimiento en nutrición. Endocrinol Nutr. 2006;53:479-83.
- Martín Moreno JM, Boyle P, Gorgojo L, Mainsonneuve P, Fernández Rodríguez JC, Salvini S, Willet WC. Development and validation of a food frequency questionnaire in Spain. Int J Epidemiol. 1993;22:512-9.
- Merson B, Pezdek K, Saywitz K. A meta-analysis of children’s self-reports of dietary intake. Psychol Health. 2017;32:186-203.





