Vol. 19 - Num. 73
Original Papers
Recent replacement by the American Academy of Pediatrics of the term “apparent life-threatening event” (ALTE) to the current “brief resolved unexplained event” (BRUE). Comments on five years’ experience in home cardiorespiratory monitoring
Miguel Ángel Zafra Anta
aServicio de Pediatría. Hospital Universitario de Fuenlabrada. Fuenlabrada. Madrid. España.
bMIR-Pediatría. Hospital Universitario de Fuenlabrada. Fuenlabrada. Madrid. España.
cPediatra. CS El Naranjo. Fuenlabrada. Madrid. España.
Correspondence: MA Zafra. E-mail: miguelzafraanta@gmail.com
Reference of this article: Zafra Anta MA, Alonso de la Hoz J, Fernández Manso B, Nieto Gabucio N. Recent replacement by the American Academy of Pediatrics of the term “apparent life-threatening event” (ALTE) to the current “brief resolved unexplained event” (BRUE). Comments on five years’ experience in home cardiorespiratory monitoring. Rev Pediatr Aten Primaria. 2017;19:23-8.
Published in Internet: 15-02-2017 - Visits: 38641
Abstract
Infants who present with a history of an acute event (an unexpected change in breathing, appearance, or behavior) reported by their caregiver represent a heterogeneous group with diverse pathophysiology. In the past, these events were termed apparent life-threatening events (ALTE). The American Academy of Pediatrics (AAP) recommends the replacement of the term ALTE with a new term: brief resolved unexplained event (BRUE). It provides an approach to patient evaluation, and management recommendations.
Objective: to describe the clinical characteristics and the evolution of patients enrolled in a CRD monitoring program in a second level hospital. We assess this work according to the new guide from the AAP.
Methods: retrospective study of all patients with indication of monitoring CRD, enrolled in a secondary level hospital, in Pediatric Neumology consultation (2010-2014). Database: Excel 2010®.
Results: seven patients were monitored (7/10,000 born), all males. The initial indication of monitoring CRD was: serious or recurrent ALTE (five cases), severe neonatal hypotonia (one case) and brother of sudden infant death (SID) (one case). The mean age of indication was 59.8 days.
Conclusions: we support the AAP proposition to use the term BRUE with or without risk factors, avoiding the name ALTE. Because of the diverse presentations, causes, risk factors, and prognosis of infants presenting with acute events, evaluation and management should be individualized. Most of the patients in which monitoring CRD is indicated may have tracking in a second level hospital. Long-term follow-up programs of infants with a BRUE-ALTE could contribute to adapt the healthcare activities to the needs of each patient and confirm the medical diagnosis.
Keywords
● Ambulatoty monitoring ● Infantile apparent life-threatening event ● Sudden infant deathINTRODUCTION
Since 1986, cases of infants presenting to emergency medical services with sudden acute episodes of altered respiration, appearance or behaviour have been classified as apparent life-threatening events (ALTEs) if caregivers had experienced the episodes as frightening.1 However, many of these events do not pose a threat to the infant’s life. For a long time, the use of this label for the purpose of diagnosis has been in question.2
The American Academy of Pediatrics (AAP) recently proposed a clinical practice guideline for paediatricians that applies to infants aged less than 1 year in which they recommend replacing the term ALTE by the alternative brief resolved unexplained event (BRUE). This guideline proposes an approach to the management of BRUE by levels of risk defined by the clinical manifestations and the presence or absence of risk factors.3 Patients need to be evaluated by a health professional to determine whether a BRUE actually took place or, for example, the infant simply experienced a normal change in colour. Under the new definition, it is the clinician, rather than the caregiver, that determines whether the event posed a threat to the infant’s life.
Many protocols reserved the working diagnosis of ALTE for cases in which the patient required intense stimulation or resuscitation measures to recover.1,4,5 Thus, the management of these infants in paediatric emergency departments conformed to specific diagnostic algorithms.6,7
Although years ago it was suggested that there could be an association between ALTE and sudden infant death syndrome (SIDS), the studies conducted in the past two decades have failed to establish a causal relationship between the two.
The infants eligible for home cardiorespiratory monitoring (CRM) are a heterogeneous group that, until recently, ranged from healthy infants with a history of SIDS in one or more siblings to infants that had experienced an ALTE and with underlying disease or risk factors (preterm birth and apnoeic episodes, recurrent ALTEs, and other). It is essential that the approach to the management of these patients be based on the characteristics of the event and a careful history and physical examination, both of which should be reassessed at a later time.
Our aim was to describe the clinical characteristics and outcomes of the patients included in the home CRM programme of a secondary level hospital, and to assess their management based on the recommendation of the new guidelines for BRUE of the AAP.
This study was presented partially at the 2015 Congress of the Asociación Española de Pediatría (Spanish Association of Pediatrics [AEP]).
METHODS
We conducted a descriptive retrospective study of all patients in whom home CRM was prescribed and whose care was managed from the paediatric pulmonology clinic of a secondary level hospital (Fuenlabrada, Madrid, Spain) over a period of five years (2010–2014). The hospital serves a catchment area with a population of 222 531 inhabitants and manages 2000 deliveries a year. We used an Excel® 2010 database.
RESULTS
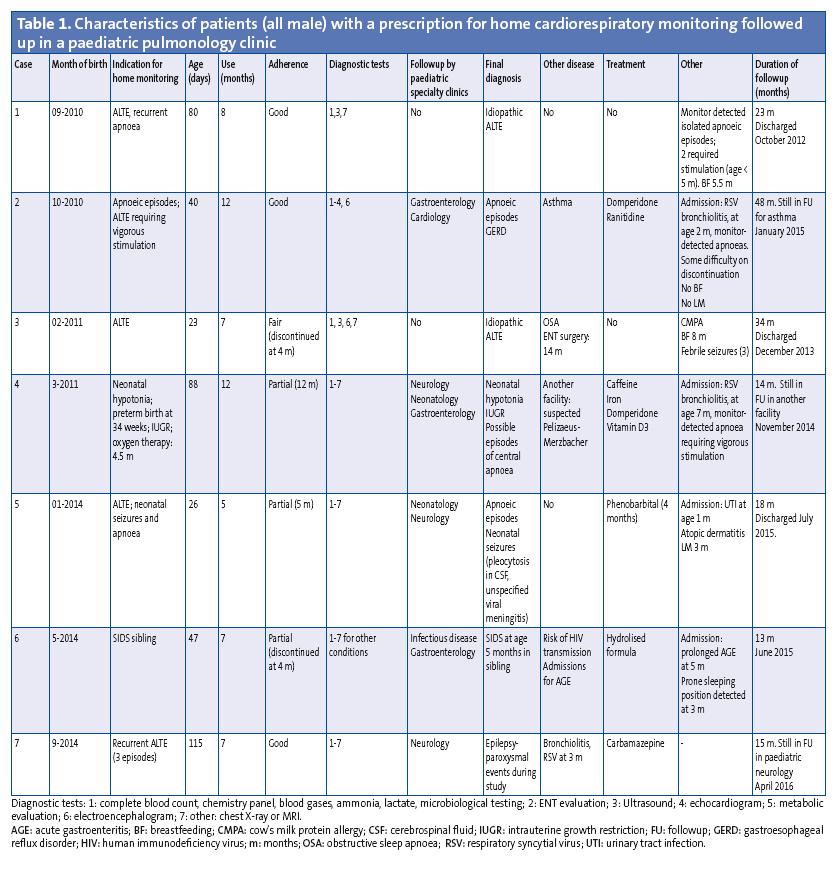
Monitoring was prescribed in seven patients (approximately 7/10 000 births), all of them male (Table 1). The initial indication for home CRM was recurring or severe ALTE (5 cases), severe neonatal hypotonia (1 case) and history of SIDS in sibling (1 case). The mean age at the time of prescription was 59.8 days. Adherence was partial in three patients, and one patient discontinued monitoring early. The final diagnosis was idiopathic ALTE in two patients. One had apnoea associated with gastroesophageal reflux. Another patient underwent monitoring due to a history of SIDS in a sibling. The rest received a diagnosis of neurologic disease.
DISCUSSION
Home CRM in our patients was prescribed on a case-to-case basis for indications previously accepted by various protocols1,5; and its setup and followup from a specialised secondary-level paediatric pulmonology clinic was feasible. Its use did not impact family quality of life significantly, although adherence was partial or poor in four cases.
When we compared the ALTE cases in our patients with the current classification of BRUE, we observed that none corresponded to cases of BRUE without risk factors. Of the five patients that had ALTEs as defined by the past terminology, three were aged less than 60 days and two experienced recurrent events or had a past history of disease (neonatal seizures).
Home monitoring was prescribed due to risk of SIDS in only one case in our series, and this patient also had a history of admission for gastroenteritis and may have had other disease risk factors.
The AAP proposes that the reason for the visit be documented as an acute event until the assessment establishes whether the criteria for BRUE are met.3,8 A brief resolved unexplained event would entail:
- Colour change (cyanosis or pallor).
- Changes in respiration such as decreased, irregular or absent breathing (apnoea).
- Marked change in tone (hypo- or hypertonia).
- Altered level of responsiveness.
The following also need to be met to fulfil the criteria for BRUE3,8: no apparent explanation for the event (respiratory infection or vomiting due to reflux), resolution prior to emergency department visit, well-appearing at the time of the visit, and event duration of less than 1 minute. Based on the current evidence, the AAP defines lower-risk infants as those meeting all of the following criteria:
- Age more than 60 days.
- Prematurity at gestational age ≥ 32 weeks and corrected age ≥ 45 weeks.
- No previous BRUE.
- Duration of BRUE of less than 1 minute
- No CPR required by trained health care provider
- No concerning features in detailed medical history and/or physical examination.
- No family history of sudden death.
Lower-risk patients required a short period of observation and few additional interventions. It is important to remember to ask about natural remedies, non-prescription medications or cough syrups and to consider the possibility of child abuse.
If infants do not meet the criteria for BRUE or have risk factors, there is a higher probability of a significant underlying aetiology or of recurrent episodes; these patients require observation and diagnostic tests to rule out underlying diseases. If a specific aetiology is suspected (gastroesophageal reflux, infection, metabolic or neurologic disorder, etc), the workup will be based on the suspected condition (Table 2).
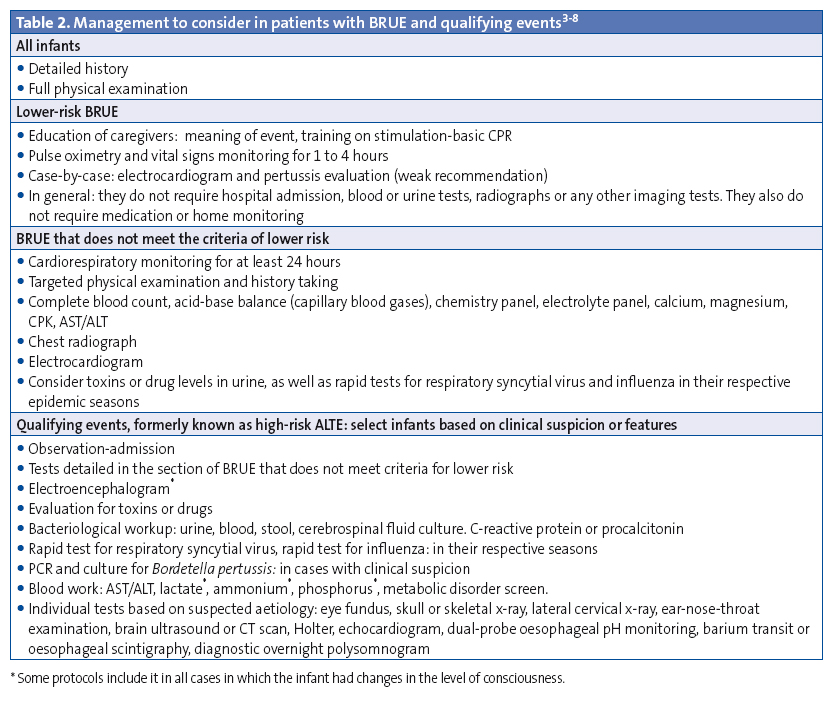
The management of patients according to the definition and guidelines for BRUE of the AAP will affect the approach followed in paediatric emergency departments, decisions regarding admission and diagnostic tests, and possibly the use of home CRM, too.
The currently established indications for home CRM are9:
- Preterm infants with persistent apnoea of prematurity and no comorbidities that can be discharged from hospital.
- Chronic pulmonary disease, tracheostomy or airway abnormalities, neurologic or metabolic diseases affecting respiratory control. In these patients, monitoring of oxygen saturation by pulse oximetry is recommended to detect events early.
- Case-by-case decision for the following: qualifying events (previously known as ALTE), depending on the suspected underlying mechanism, or patients considered to be at risk of recurrent events, when monitor recordings could provide information on the development of clinically relevant events or episodes of central apnoea.
Cardiorespiratory monitoring (heart rate and chest wall movements) is more useful in detecting non-obstructive apnoeas in the absence of bradycardia or pulse oximetry desaturation. There is no evidence at present that home CRM can help prevent sudden infant death syndrome in asymptomatic infants, including those with a sibling history of SIDS,9 although the decision to use monitoring can be made on a case-to-case basis, taking into account its risks and benefits.
CONCLUSIONS
We concur with the proposal of discontinuing the use of the term ALTE and using the new alternative of BRUE with or without risk factors. Due to the variability in the presentation, causes, risk factors and outcomes in infants that experience an acute event, the approach to their diagnosis must be individualised.
Most patients in whom the use of home CRM is considered could be followed up from secondary care hospitals in coordination with other paediatric specialties depending on the underlying disease.
Long-term follow-up programmes for infants with characteristic events, formerly known as ALTEs, can help tailor health care interventions to the individual needs of each patient, and to confirm the clinical diagnosis.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article. This study was presented partially at the 2015 Congress of the AEP.
ABBREVIATIONS: AAP: Americana Academy of Pediatrics · ALTE: apparent life-threatening event · BRUE: brief resolved unexplained event · CRM: cardiorespiratory monitoring · SIDS: sudden infant death syndrome.
REFERENCES
- Izquierdo Macián MI. Libro Blanco de la Muerte Súbita Infantil. Monografías de la AEP. 3.ª edición. Madrid: Ergon; 2013.
- Esparza Olcina MJ. Abordaje de los episodios aparentemente letales en lactantes: revisión sistemática. Evid Pediatr. 2014;10:75.
- Tieder JS, Bonkowsky JL, Etzel RA, Gremse DA, Herman B, Katz ES, et al. Brief resolved unexplained events (formerly apparent life-threatening events) and evaluation of lower-risk infants. Pediatrics. 2016;137:e20160590.
- Sánchez Etxaniz J, Santiago Burruchaga M, González Hermosa A, Rodríguez Serrano R, Astobiza Beobide E, Vega Martín MI. Características epidemiológicas y factores de riesgo de los episodios aparentemente letales. An Pediatr (Barc). 2009;71:412-8.
- Zafra Anta MA, Nieto Gabucio N. Síndrome de muerte súbita del lactante. In: López-Herce Cid J, Calvo Rey C, Rey Galán C, Rodríguez Núñez A, Baltodano Agüero A. Manual de Cuidados Intensivos Pediátricos. 4.ª edición. Madrid: Publimed; 2013. p. 196-200.
- Leal J, García M. Evaluación y seguimiento de lactantes que sufrieron un episodio aparentemente letal. An Pediatr Contin. 2010;8:98-103.
- Santiago Burruchaga M, Sanchez Etxaniz J, Benito Fernández J, Vázquez-Cordero C, Mintegi-Raso S, Labayru-Echeverría M, et al. Assessment and management of infants with apparent life-threatening events in the paediatric emergency department. Eur J Emerg Med. 2008;15:203-8.
- Corwin MJ, Acute events in infancy including brief resolved unexplained event (BRUE). In: UpToDate [online] [updated 11/10/2016, accessed 10/02/2017]. Available at www.uptodate.com/contents/acute-events-in-infancy-including-brief-resolved-unexplained-event-brue
- Corwin MJ. Use of home cardiorespiratory monitors in infants. In: UpToDate [online] [updated 28/06/2015, accessed 10/02/2017]. Available at www.uptodate.com/contents/use-of-home-cardiorespiratory-monitors-in-infants





