Vol. 17 - Num. 68
Original Papers
Relief of pain and distress during immunizations. Synthesis of evidence. Recommendations of the Advisory Committee on Vaccines of the Spanish Association of Paediatrics
Nuria García Sáncheza, Manuel Merino Moínab, César García Verac, I Lacarta Garcíad, L Carbonell Muñoze, Beatriz Pina Marquésf, FJ Álvarez Garcíag, J Arístegui Fernándezh, en nombre del Comité Asesor de Vacunas de la Asociación Española de Pediatría (CAV-AEP)
aPediatra. CS Delicias Sur. Zaragoza. España.
bPediatra. CS El Greco. Getafe. Madrid. España.
cPediatra. CS José Ramon Muñoz Fernández. Zaragoza. España.
dMIR-Anestesiología, Reanimación y Terapia del dolor. Hospital Universitario Miguel Servet. Zaragoza. España.
eEnfermera de Pediatría. CS Parque Coimbra. Móstoles. Madrid. España.
fMatrona. Hospital Universitario Miguel Servet. Zaragoza. España.
gPediatra. CS de Llanera. Departamento de Medicina. Universidad de Oviedo. Asturias. España.
hUnidad de Infectología Pediátrica. Hospital Universitario de Basurto. Departamento de Pediatría. Facultad de Medicina de la Universidad del País Vasco (UPV/EHU). Bilbao. España.
Correspondence: N García. E-mail: nuriagarciasanchez4@gmail.com
Reference of this article: García Sánchez N, Merino Moína M, García Vera C, Lacarta García I, Carbonell Muñoz L, Pina Marqués B, et al. Relief of pain and distress during immunizations. Synthesis of evidence. Recommendations of the Advisory Committee on Vaccines of the Spanish Association of Paediatrics. Rev Pediatr Aten Primaria. 2015;17:317-27.
Published in Internet: 19-11-2015 - Visits: 91054
Abstract
Background: in healthy children and adolescents, immunizations that require a needle related procedure are the most common source of pain and distress. Parents, children, adolescents and health-care providers are concerned about this. The Advisory Committee on Immunization of the Spanish Association of Pediatrics (CAV-AEP) believes that address pain and distress at the time of vaccination is necessary following recommendations that have to be based on rigor and science.
Methods: we divided the subject in four areas: Breastfeeding and oral sucrose solutions, topical anaesthetics, vaccination administration methods and other interventions (distraction). Synthesis of evidence was made. Assuming the recommendations of The Clinical Guideline of Anne Taddio (2010) and adding the evidence of clinical trial published after the Guide.
Results: methods that showed effectiveness in diminishing pain were: for infants, breastfeeding before, during and after the puncture is effective in manage pain. Oral sucrose solutions could be an alternative if breastfeeding is not possible. Topical anaesthetics are effective for all ages but a time to produce effect is required and need financial resources. No aspiration for intramuscular injection, put the injection as quickly as possible, give the vaccines so that the most painful the last. If more than one vaccine injection are required in the same visit, and it is possible, it is preferable to inject simultaneously more than one vaccine than sequentially. Hold the infant. For children 2 to 14 years use distraction techniques.
Conclusions: as a thorough revision of the topic was made, there are enough evidence to recommend that in any setting where children immunization is given, techniques to mitigate pain at the time of vaccination should be implemented, moreover these strategies are simple and easy to assimilate in clinical practice.
Keywords
● Anesthesia and analgesia ● Immunization ● Pain ● Pain management ● VaccinationINTRODUCTION
Issues related to pain in young children have not received much attention in past decades, probably due to misconceptions regarding the perception of pain and a lack of knowledge of analgesic and anaesthetic techniques that have led to routinely neglecting pain during child vaccination.1 Rigorous research has demonstrated that infants have the anatomical and functional capacity to perceive pain,2 and tissue changes in response to noxious events have been observed that could be interpreted as responses to pain.3,4
Vaccine administration is the painful procedure most frequently performed in healthy children. Failure to adequately manage pain during vaccination exposes children to unnecessary suffering and may have long-term consequences, such as a fear of needles and of health care.5 Children aged 4 to 14 years express their wish to be prepared ahead of time and request measures to reduce pain during vaccination.6
Many publications have addressed this issue, but few professionals have integrated their recommendations to their everyday practice due to ignorance or misconceptions. The dissemination of these techniques and the training of health providers have led to an increase in their use, greater satisfaction in health professionals, patients and families, and improved adherence to the childhood immunisation schedule.7-9
The Advisory Committee on Vaccines of the Spanish Association of Paediatrics (Comité Asesor de Vacunas de la Asociación Española de Pediatría [CAV-AEP]) felt the need to have a multidisciplinary team develop an evidence-based guideline or set of recommendations for the management of pain during child vaccination. The objectives pursued in this endeavour are to reduce the distress involved in the act of vaccination, to make vaccination more humane, to achieve greater adherence to childhood immunisation schedules, and to reduce the long-term psychological sequelae that result from negative experiences with pain.
MATERIALS AND METHODS
Methods used in the review and summary of evidence
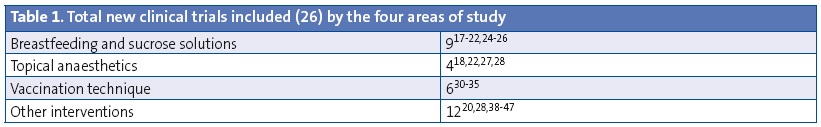
To produce this document, the team responsible for it divided into four sections the aspects related to pain caused by vaccination procedures that were going to be analysed (breastfeeding or ingestion of sucrose solutions, topical anaesthetics, other physical methods including distractions, and injection techniques). The team assumed that the recommendations of the evidence-based clinical practice guideline of Anna Tadio5 and of the Cochrane systematic reviews on these subjects were valid due to the high quality of these sources.10-15 Our team performed searches in the following electronic databases, starting from the end dates of the searches of the aforementioned guideline and systematic reviews: TripDatabase, Cochrane, Epistemonikos, Cinhal, Centre for Review Dissemination, PubMed, Embase, Biblioteca Virtual en Salud and Sumarios IME and CUIDEN (ending in February 2015). We did not apply any language restrictions, but we only reviewed randomised controlled or non-controlled clinical trials. We also reviewed the references in these articles. We ultimately retrieved 27 articles (Table 1): nine on breastfeeding and sucrose solutions, four on topical anaesthetics, twelve on other methods, and six on vaccination technique. We evaluated the risk of bias of the selected articles by means of the tool proposed by the Cochrane Collaboration.16
We used the GRADE system (Tables 2 and 3) to classify the evidence and grade the strength of the recommendations.17 The GRADE system offers only two grades for the strength of recommendation:
- Strong: there is a high degree of confidence that the desirable effects of the intervention outweigh the undesirable effects (strong recommendation in favour) or vice versa (strong recommendation against).
- Weak: the desirable effects of the intervention probably outweigh its undesirable effects (weak recommendation in favour) or vice versa (weak recommendation against), but there is a lower degree of certainty.
The recommendations that were not clear after applying this system but on which the reviewing team reached a consensus are presented as such (recommendations based on author consensus).


RESULTS
Breastfeeding and ingestion of sweet-tasting solutions
Amamantamiento
Breastfeeding is the natural and optimal method for feeding young children. Breastfeeding mothers intuitively offer the breast to their babies to provide not only nourishment, but also relief when there is pain or disease, and the infant seeks the mother’s breast when in need of comfort.
There is clear and robust scientific evidence that breastfeeding, compared to placebo or non-intervention, reduces the signs of pain resulting from simple painful procedures (venipuncture, intramuscular injection, heel puncture, etc) performed on young infants. As an analgesic, breastfeeding is superior to the direct administration of human milk or sweet-tasting solutions, the use of a pacifier while holding the child, and other nonpharmacological pain-relief strategies.5-10,17-21 There is also evidence of its synergistic interaction with topical analgesic medication.22
Breastfeeding is considered a combined analgesic technique, as it encompasses distraction through sucking, the release of endogenous opioids due to the perception of sweetness, skin-to-skin contact and a stress-relieving effect through the release of oxytocin and possibly melatonin.
On the other hand, when it comes to the administration of oral live vaccines in a single vaccination event, recent studies have not found an association between breastfeeding around the time of vaccination against rotavirus and the resulting immunogenicity.23
As for optimal technique, it is important that enough time is provided for achieving an effective latch on before the painful procedure is performed. Breastfeeding should proceed through the administration of injectable vaccines and ideally continue after.
There have been no reports of adverse effects, such as choking, resulting from this practice. The ergonomic challenges that may arise from administering vaccines while the child is being breastfed are minor and vastly compensated by the benefits of this practice.
Recommendation 1: We recommend breastfeeding infants during vaccination as the best method for analgesia and comfort (strong recommendation in favour).
Sweet-tasting solutions
Oral administration of dextrose or sucrose is a useful and safe analgesic method that is commonly implemented during performance of painful procedures in neonates.11 To date, more than one hundred clinical trials have compared the administration of sweet-tasting substances to other interventions and placebo in different paediatric age groups.
In addition to providing a distraction, the analgesic effect of sucrose administration seems to be mediated by taste receptors in the mouth and produced by the release of endogenous opioids by the midbrain.
The analgesic potency of performing this intervention ahead of painful stimuli—such as the injection of vaccines—is associated with age, and it is highest in newborns, appreciable in infants less than 12 months of age, and less evident after age 1 year.5,11-13,24,25 However, a recent study had statistically significant results supporting its use in children aged 16 to 18 months, especially for high concentrations of sucrose (75%).26
This method can be combined with sucking on a pacifier, but is not recommended for pain management under other circumstances.
The optimal schedule, concentration and volume of administration remains to be established, but we recommend its administration one to two minutes prior to the injection. The most commonly used schedule consists of a single dose of 12 to 25 g of sucrose in 10 mL of water, depending on the child’s age.5,17
Recommendation 2: if breastfeeding is not an option, and in children aged up to 18 months, we recommend the administration of an oral sweet solution of sucrose in water prior to vaccine injection (strong recommendation in favour).
Topical anaesthetics
Pain management during child vaccination requires the combination of different techniques due to their synergistic effect. The use of a topical anaesthetic, alone or combined with other methods, is very effective. Chief among the anaesthetics used are EMLA® cream, a mixture of topical lidocaine and prilocaine (2.5%); lidocaine cream (Lambdalina®); and ethyl chloride cold spray (Cloretilo Chemirosa®). EMLA® is the one used most frequently, as it can be administered as early as the neonatal period, while the minimum age for Lambadalina® is 6 years and the evidence on ethyl chloride is insufficient (only one study, which showed that it was less efficacious than breastfeeding in infants aged less than 6 months).18
Several studies have demonstrated the clear benefits of using these drugs for pain relief during vaccination.18,22 Some scientific associations include the use of topical anaesthetics in their vaccination guidelines (essentially the EMLA® cream).5,14 There is evidence of a statistically significant decrease in internationally validated pain scales, such as the Visual Analog Scale (VAS)27 and other factors such as behavioural changes, the duration of crying or scores based on facial expressions.28
The salient features of the EMLA® cream are its high safety profile and the need to apply it one hour prior to injection to achieve the desired anaesthetic effect. The effectiveness of this product is due to its good penetration, of up to 5 mm, which provides a superficial anaesthesia that is effective enough even for minor surgeries. While other techniques have poorer results when applied to older children (breastfeeding, sucrose solutions), the effectiveness of topical anaesthetics is sustained across all ages. The amount required (the size of a coin, covered with a bandage) is not associated with methaemoglobinaemia.
Recommendation 3: Administration of a topical anaesthetic, for instance a cream like EMLA®, sufficiently ahead of time, is recommended for the prevention of vaccination-related pain in all paediatric age groups (strong recommendation in favour).
Vaccine administration technique
This section refers to all puncture-related procedures used for vaccine administration.
Brand of vaccine
In some cases, there are different formulations for a single vaccine. Different preparations that use the same antigens may cause different amounts of pain.5,29
Recommendation 4: elegir la marca de vacuna menos dolorosa, cuando sea posible (recomendación fuerte a favor).
Position of child
The supine position results in more pain than the child being held by a parent5 or in skin-to-skin contact during the neonatal period.30 Excessive restraining must be avoided so as to not increase the fear of the child.
Recommendation 5: avoid the supine position (strong recommendation in favour).
Injection technique
The evidence shows that aspiration before injection and slow injection of vaccines result in increased pain.5,31
Recommendation 6: inject intramuscular vaccines quickly and without prior aspiration (strong recommendation in favour).
Order of vaccine injections
There are times that two or more vaccines have to be administered during a single visit. Since some vaccines are more painful than others, and pain may increase with each injection, the order in which vaccines are administered may affect the pain response.5,32,33
Recommendation 7: when administering multiple vaccines sequentially, the most painful vaccine should be injected last (weak recommendation in favour).
Routes of administration
Some vaccines can be administered by either the intramuscular or the subcutaneous route. At present, there is no evidence of either route causing less pain, but it appears that the subcutaneous route is associated with a higher risk of subsequent reactivity.
Recommendation 8: Ensure that intramuscular injections reach the appropriate depth. This does not change the amount of pain at the time of injection, but it may improve the physical sensations after vaccination (recommendation based on the consensus of the authors).
Multiple injections
The simultaneous administration of vaccines by two individuals has been proposed as an efficacious method for pain reduction in infants.34,35
Recommendation 9: In infants, administer vaccines simultaneously as opposed to sequentially if enough staff are available (weak recommendation in favour).
Temperature
The available evidence does not support warming up for the purpose of reducing pain during their administration,7,36 although rubbing the vaccine between both hands guarantees a more homogeneous suspension of the vaccine components.
Recommendation 10: rub the vaccine between both hands prior to administration (recommendation based on the consensus of the authors).
Injection site
Selection of the appropriate site for vaccine administration (for nonwalking children, the middle third of the vastus lateralis, and the deltoids for all others) guarantees immunogenicity and reduces the incidence of adverse reactions.37
Recommendation 11: choose the injection site that will guarantee the administration by the indicated route based on the age and characteristics of the child (weak recommendation in favour).
Needle size
In vaccines requiring intramuscular administration, injection with short needles results in the delivery of vaccine contents to the subcutaneous tissue, which is associated with a higher incidence of adverse effects, such as local reactivity and pain.36,37
Recommendation 12: choose a needle that is long enough to penetrate the muscle, based on the site of administration and the age and characteristics of the child (weak recommendation in favour).
Other interventions
This section includes all techniques other than the ones previously described, labelled by some authors as psychological interventions, nonpharmacological interventions, etc.
Distraction techniques
Some authors describe distraction as one of the key interventions for managing pain during vaccination.36 It has been hypothesised that diverting the recipient’s attention to stimuli unrelated to vaccination may affect the processing and perception of pain, and neurophysiological studies have demonstrated that the areas of the brain involved in the processing of pain stimuli appear less active during performance of distraction tasks.
Psychological interventions that address needle-related procedural pain and distress have been studied in detail, and are the subject of an extensive Cochrane review14 and an earlier systematic review.38 Studies in this area have focused on children aged 2 to 19 years that had to undergo needle-related procedures, including vaccination. To summarise, we want to highlight that the review by Uman and subsequent clinical trials included in our review provide evidence that distraction techniques are effective in the management of procedural pain and distress, especially in children aged less than 12 years,14,28,39-43 but other psychological interventions have not been sufficiently researched.14
A subsequent systematic review by Chambers et al38 corroborated the efficacy of simple interventions, such as long and slow breathing exercises, distraction techniques directed by the child or the nursing professional using age-appropriate devices and methods, and cognitive-behavioural interventions to relieve pain and distress during vaccination. The distraction techniques they proposed are simple: reading a story, listening to music, watching a screen, and generally driving the child’s attention to anything other than the injection. The use of electronic devices such as tablets, game consoles or music players has been described and is particularly indicated in adolescents. There are few studies on the population aged more than 14 years, but a randomised clinical trial found that vaccination pain was reduced by listening to music, even without headphones.44 We must underscore that all these interventions are simple, do not require significant expenditures, and are very beneficial. If toys are used, they should be cleaned properly between users so they do not act as fomites.
Recommendation 13: In children aged 2 to 19 years, use distraction techniques, such as reading a story or playing music (strong recommendation in favour). In adolescents aged 14 years, play music without using headphones (weak recommendation in favour).
Tactile stimulation
Caressing, rubbing or applying pressure to the skin around the injection site is a cost-neutral intervention that may reduce the pain of vaccination. It should be performed before and during vaccine injection, but not after, as the latter could promote reactivity. This technique is based on the hypothesis that the sensation of touch competes with the sensation of receiving an injection, reducing the perception of pain. Since there are few studies on young children and tactile stimulation could elicit an unpleasant sensation in this subset of the population, these techniques are recommended for children aged more than 4 years, for whom quasi-experimental data are available.5 In newborns and infants, tactile stimulation should be very gentle and accompanied by other techniques in a “sensory saturation” approach, which consists in providing multisensory stimulation through touch, taste, hearing and vision, for instance by talking to the child, caressing the child’s face and feeding a sweet-tasting solution.20,42,45-47
Recommendation 14: in children aged 4 or more years, rub or caress the skin around the injection site with moderate intensity before and during administration (weak recommendation in favour).
DISCUSSION
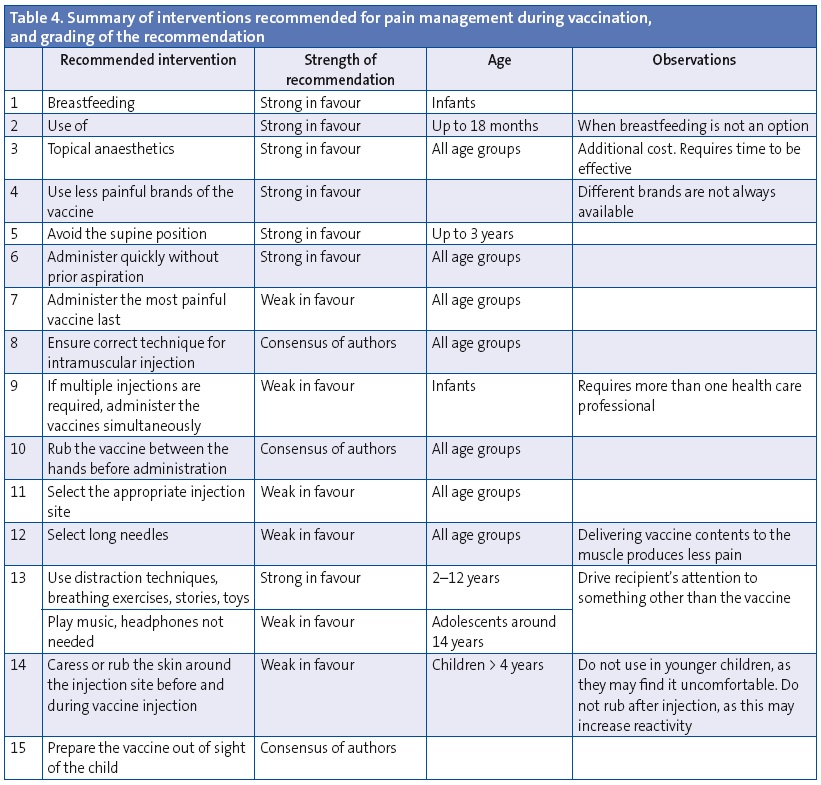
To conclude, based on the available evidence we believe that all techniques that have been proven effective in the management of pain and suffering during vaccination (Table 4) should be used. Considering the efficacy demonstrated by these strategies, there is no justification for neglecting this issue.
The complexity of immunisation schedules continues to grow as new safe and efficacious vaccines become available. While publications on techniques that may facilitate pain management abound, it is uncommon for professionals in Spain to have integrated these procedures to their everyday practice. Our aim was for this document to provide a summary of the available evidence in order to learn which techniques have sufficient scientific support to be recommended. Our set of recommendations is comprised exclusively of recommendations based on clinical trials conducted after the clinical practice guideline by Taddio5 and the Cochrane systematic reviews9-14 on these subjects, the conclusions of which we had accepted from the outset.
There are procedures that we consider good clinical practice and actually recommend for which there was no supportive evidence at this time, which we consequently presented as based on the authors’ consensus, such as warming the vaccine by rubbing it between the hands, having the health provider prepare the vaccine out of sight of the recipient (recommendation 15), and ensuring that intramuscular injections are deep enough.
When we had concluded our literature search and the evidence summary, we were pleased to learn that the World Health Organization had recommended that pain-relief techniques be used whenever vaccines are administered.48 Recently, A. Taddio has published a new clinical practice guideline49 with updated information that extends the recommendations to the entire lifespan, as it also applies to adults. As we did in the present work, A. Taddio and her team used the GRADE system to establish their recommendations. We are encouraged by the fact that our review, conducted concurrently and without knowledge of their research, has led to very similar results. The results diverge minimally, for instance, in the recommendation to administer the most painful vaccine last, which we graded as a weak in favour recommendation and they as a strong in favour recommendation. Taddio’s guideline graded the use of distraction interventions as weak in favour, while we graded it as strong in favour. Furthermore, their guideline strongly recommends educating professionals, parents and children older than 3 years on pain management during vaccination. In the section devoted to sweet-tasting solutions, they propose the use of the oral formulation of the rotavirus vaccine as an alternative in children scheduled for rotavirus vaccination.50
Our work is not complete with the writing of this article. We must endeavour to convey to paediatricians and nursing professionals that the administration of vaccines with concurrent management (alleviation) of pain and stress constitutes excellent clinical practice. This only takes simple training, achieved just by reading these recommendations, and generally does not require additional expenditures or time. Furthermore, health providers that implement these measures generally report greater satisfaction, while such an implementation may facilitate an improved adherence to the immunisation schedule.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare in relation to the preparation and publication of this article.
ABREVIATURAS: CAV-AEP: Advisory Committee on Vaccines of the Asociación Española de Pediatría (Spanish Association of Pediatrics).
ACKNOWLEDGMENTS
We want to thank Ángel Hernández Merino, for his contributions to the writing of the article, and the CAV-AEP for their decisive support for the project overall.
REFERENCES
- Taddio A, Chambers C, Halperin S, Ipp M, Lockett D, Rieder MJ, et al. Inadequate pain management during childhood immunization: the nerve of it. Clin Ther. 2009;31:S152-67.
- Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507-20.
- Slater R, Cornelissen L, Fabrizi L, Patten D, Yoxen J, Worley A. Oral sucrose as an analgesic drug for procedural pain in newborn infants: a randomized controlled trial. Lancet. 2010;376:1225-32.
- Grunau R, Craig KD. Pain expression in neonates: facial action and cry. Pain. 1987;28:395-410.
- Taddio A, Appleton M, Bortolussi R, Chambers C, Dubey V, Halperin S, et al. Reducing the pain of childhood vaccination: an evidence-based clinical practice guidelines (summary). CMAJ. 2010;182:e843-e55.
- Taddio A, Ilersich AF, Llersich AN, Wells J. From the mouth of babes: getting vaccinated doesn’t have to hurt. Can J Infect Dis Med Microbiol. 2014;25:196-200.
- Schechter NL, Bernstein BA, Zempsky WT, Bright NS, Willard AK. Educational outreach to reduce immunization pain in office settings. Pediatrics. 2010;126:e1514-21.
- Chan S, Pielak K, McIntyre C, Deeter B, Taddio A. Implementation of a new clinical practice guideline regarding pain management during childhood vaccine injections. Paediatr Child Health. 2013;18:367-72.
- Pillai Riddell RR, Racine NM, Turcotte K, Uman LS, Horton RE, Din Osmun L, et al. Non-pharmacological management of infant and young child procedural pain.Cochrane Database Syst Rev. 2011;(10):CD006275.
- Shah PS, Herbozo C, Aliwalas LL, Shah VS. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database Syst Rev. 2012;12:CD004950.
- Stevens B, Yamada J, Lee GY, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev. 2013;1:CD001069.
- Kassab M, Foster JP, Foureur M, Fowler C. Sweet-tasting solutions for needle-related procedural pain in infants one month to one year of age. Cochrane Database Syst Rev. 2012;12:CD008411.
- Harrison D, Yamada J, Adams-Webber T, Ohlsson A, Beyene J, Stevens B. Sweet tasting solutions for reduction of needle-related procedural pain in children aged one to 16 years. Cochrane Database Syst Rev. 2015;5:CD008408.
- Uman LS, Birnie KA, Noel M, Parker JA, Chambers CT, McGrath PJ, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;10:CD005179.
- Higgins JPT, Green S (eds.). Manual Cochrane de Revisiones Sistemáticas de Intervenciones, versión 5.1.0. In: Cochrane Iberoamérica [online] [updated in 2011 March, consulted on 19/11/2015]. Available in http://es.cochrane.org/sites/es.cochrane.org/files/uploads/Manual_Cochrane_510_reduit.pdf
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-6.
- McNair C, Campbell Yeo M, Johnston C, Taddio A. Nonpharmacological management of pain during common needle puncture procedures in infants: current research evidence and practical considerations. Clin Perinatol. 2013;40:493-508.
- Boroumandfar K, Khodaei F, Abdeyazdan Z, Maroufi M. Comparison of vaccination-related pain in infants who receive vapocoolant spray and breastfeeding during injection. Iran J Nurs Midwifery Res. 2013;18:33-7.
- Modarres M, Jazayeri A, Rahnama P, Montazeri A. Breastfeeding and pain relief in full-term neonates during immunization injections: a clinical randomized trial.BMC Anesthesiol. 2013;13:22.
- Esfahani MS, Sheykhi S, Abdeyazdan Z, Jodakee M, Boroumandfar K. A comparative study on vaccination pain in the methods of massage therapy and mothers' breast feeding during injection of infants referring to Navabsafavi Health Care Center in Isfahan. Iran J Nurs Midwifery Res. 2013;18:494-8.
- Iqbal A, Malik R, Siddique M, Yaqub M, IqbalT, Farrukh H, et al. Breast feeding of pain relief during Bacillus Calmette Guerin (BCG) vaccination in term neonates. Pakistan J Med Health Sci. 2014;8:403-6.
- Gupta NK, Upadhyay A, Agarwal A, Goswami G, Kumar J, Sreenivas V. Randomized controlled trial of topical EMLA and breastfeeding for reducing pain during wDPT vaccination. Eur J Pediatr. 2013;172:1527-33.
- Rongsen-Chandola T, Strand TA, Goyal N, Flem E, Rathore SS, Arya A, et al. Effect of withholding breastfeeding on the immune response to a live oral rotavirus vaccine in North Indian infants. Vaccine. 2014;32:A134-9.
- Curry DM, Brown C, Wrona S. Effectiveness of oral sucrose for pain management in infants during immunizations. Pain Manag Nurs. 2012;13:139-49.
- Goswami G, Upadhyay A, Gupta NK, Chaudhry R, Chawla D, Sreenivas V. Comparison of analgesic effect of direct breastfeeding, oral 25% dextrose solution and placebo during 1st DPT vaccination in healthy term infants: a randomized, placebo controlled trial. Indian Pediatr. 2013;50:649-53.
- Yilmaz G, Caylan N, Oguz M, Karacan CD. Oral sucrose administration to reducepain response during immunization in 16-19-month infants: a randomized, placebo-controlled trial. Eur J Pediatr. 2014;173:1527-32.
- Abuelkheir M, Alsourani D, Al-Eyadhy A, Temsah MH, Meo SA, Alzamil F. EMLA© cream: a pain-relieving strategy for childhood vaccination. J Int Med Res. 2014;42:329-36.
- Boivin JM, Poupon-Lemarquis L, Iraqi W, Fay R, Schmitt C, Rossignol P. A multifactorial strategy of pain management is associated with less pain in scheduled vaccination of children. A study realized by family practitioners in 239 children aged 4-12 years old. Fam Pract. 2008;25:423-9.
- Knutsson N, Jansson UB, Alm B. Immediate injection pain in infants aged 18 months during vaccination against measles, mumps and rubella with either Priorix or MMR-II. Vaccine. 2006;24:5800-5.
- Kostandy R, Anderson GC, Good M. Skin-to-skin contact diminishes pain from hepatitis B vaccine injection in healthy full-term neonates. Neonatal Netw. 2013;32:274-80.
- Girish GN, Ravi MD. Vaccination related pain: comparison of two injection techniques. Indian J Pediatr. 2014;81:1327-31.
- Sánchez-Molero Martín MP, del Cerro Gutiérrez AM, Galán Delgado H, Muñoz Camargo JC. Respuesta al dolor de lactantes, según el orden de administración de las vacunas. Rev Enferm. 2014;37:50-7.
- Ipp M, Parkin PC, Lear N, Goldbach M, Taddio A. Order of vaccine injection and infant pain response. Arch Pediatr Adolesc Med. 2009;163:469-72.
- McGowan A, Cottrell S, Roberts R, Lankshear A. Minimising pain response during routine infant immunization. Community Pract. 2013;86:24-8.
- Hanson D, Hall W, Mills LL, Au S, Bhagat R, Hernandez M, et al. Comparison of distress and pain in infants randomized to groups receiving standard versus multiple immunizations. Infant Behav Dev. 2010;33:289-96.
- Schechter N, Zempsky W, Cohen L, McGrath PJ, McMurtry CM, Bright NS. Pain reduction during pediatric immunizations: evidence-based review and recommendations. Pediatrics. 2007;119:e1184-98.
- Comité Asesor de Vacunas de la AEP. Manual de vacunas online de la AEP: El acto de la vacunación; antes, durante y después. In: CAV-AEP [online] [updated in 2014 november, consulted on 19/11/2015]. Available in http://vacunasaep.org/printpdf/documentos/manual/cap-5
- Chambers CT, Taddio A, Uman LS, McMurtry CM, HELPinKIDS Team. Psychological interventions for reducing pain and distress during routine childhood immunizations: a systematic review. Clin Ther. 2009;31:S77-103.
- Harrington JW, Logan S, Harwell C, Gardner J, Swingle J, McGuire E, et al. Effective analgesia using physical interventions for infant immunizations. Pediatrics. 2012;129:815-22.
- Beran TN, Ramirez-Serrano A, Vanderkooi OG, Kuhn S. Reducing children's pain and distress towards flu vaccinations: a novel and effective application of humanoid robotics. Vaccine. 2013;31:2772-7.
- Shahid R, Benedict C, Mishra S, Mulye M, Guo R. Using iPads for distraction to reduce pain during immunizations. Clin Pediatr (Phila). 2015;54:145-8.
- Gray L, Garza E, Zageris D, Heilman KJ, Porges SW. Sucrose and warmth for analgesia in healthy newborns: an RCT. Pediatrics. 2015;135:e607-14.
- Hillgrove-Stuart J, Pillai Riddell R, Horton R, Greenberg S. Toy-mediated distraction: clarifying the role of agent of distraction and preneedle distress in toddlers.Pain Res Manag. 2013;18:197-202.
- Kristjánsdóttir O, Kristjánsdóttir G. Randomized clinical trial of musical distraction with and without headphones for adolescents' immunization pain. Scand J Caring Sci. 2011;25:19-26.
- Taddio A, Ho T, Vyas C, Thivakaran S, Jamal A, Ilersich AF, et al. A randomized controlled trial of clinician-led tactile stimulation to reduce pain during vaccination in infants. Clin Pediatr (Phila). 2014;53:639-44.
- Hogan ME, Probst J, Wong K, Riddell RP, Katz J, Taddio A. A randomized-controlled trial of parent-led tactile stimulation to reduce pain during infant immunization injections. Clin J Pain. 2014;30:259-65.
- Nakashima Y, Harada M, Okayama M, Kajii E. Analgesia for pain during subcutaneous injection: effectiveness of manual pressure application before injection. Int J Gen Med. 2013;6:817-20.
- Meeting of the Strategic Advisory Group of Experts on immunization, April 2015: conclusions and recommendations. Wkly Epidemiol Rec. 2015;90:261-78.
- Taddio A, McMurtry CM, Shah V, Riddell RP, Chambers CT, Noel M, et al. Reducing pain during vaccine injections: clinical practice guideline. CMAJ. 2015;187:975-82.
- Taddio A, Flanders D, Weinberg E, Lamba S, Vyas C, Ilersich AF, et al. A randomized trial of rotavirus vaccine versus sucrose solution for vaccine injection pain.Vaccine. 2015;33:2939-43.





