Vol. 16 - Num. 61
Consensus document
Immunisation schedule of the Spanish Association of Paediatrics: 2014 recommendations
David Moreno Péreza, FJ Álvarez Garcíab, J Arístegui Fernándezc, M.ª José Cilleruelo Ortegad, JM Corretger Rauet, Nuria García Sánchezf, Ángel Hernández Merinog, Teresa Hernández-Sampelayo Matosh, M Merino Moina, L Ortigosa del Castilloi, J Ruiz-Contreras, Comité Asesor de Vacunas de la Asociación Española de Pediatría
aInfectología Pediátrica e Inmunodeficiencias. Unidad de Gestión Clínica de Pediatría. Hospital Materno-Infantil. Hospital Regional Universitario de Málaga. Grupo de Investigación IBIMA. Facultad de Medicina. Universidad de Málaga. Málaga. España.
bPediatra. CS de Llanera. Departamento de Medicina. Universidad de Oviedo. Asturias. España.
cUnidad de Infectología Pediátrica. Hospital Universitario de Basurto. Departamento de Pediatría. Facultad de Medicina de la Universidad del País Vasco (UPV/EHU). Bilbao. España.
dServicio de Pediatría. Hospital Universitario Puerta de Hierro-Majadahonda. Facultad de Medicina. Universidad Autónoma de Madrid. Madrid. España.
fPediatra. CS Delicias Sur. Zaragoza. España.
gPediatra de Atención Primaria. Madrid. Vocal del Comité Asesor de Vacunas de la AEP. España.
hServicio de Pediatría. Hospital General Universitario Gregorio Marañón. Facultad de Medicina. Universidad Complutense de Madrid. Madrid. España.
iServicio de Pediatría. Hospital Universitario Ntra. Sra. de Candelaria. Facultad de Medicina. Universidad de La Laguna. Tenerife. España.
Correspondence: D Moreno. E-mail: dmp.malaga@gmail.com
Reference of this article: Moreno Pérez D, Álvarez García FJ, Arístegui Fernández J, Cilleruelo Ortega MJ, Corretger Rauet JM, García Sánchez N, et al. Immunisation schedule of the Spanish Association of Paediatrics: 2014 recommendations. Rev Pediatr Aten Primaria. 2014;16:13-20.
Published in Internet: 10-02-2014 - Visits: 61591
2014 RECOMMENDATIONS
The Advisory Committee on Vaccines of the Spanish Association of Paediatrics (CAV-AEP) updates the immunisation schedule yearly taking into account epidemiological aspects as well as the safety, efficacy and efficiency of vaccines. These recommendations are addressed to paediatricians, family physicians, nursing staff, midwives, children’s family members, and more generally to anyone interested in having updated information on immunisations in the paediatric age group.
The current schedule for 2014 (Table 1) continues to include grades of recommendation. We have defined as routine vaccinations those which the CAV-AEP considers should be given systematically to all children in Spain; as recommended those which present a profile for routine immunisation in the paediatric age group and which the CAV-AEP would like to have given to all children, but whose priority must be assessed as a function of the economic resources available for its public funding due to cost-efficiency issues; and as high-risk group vaccinations those indicated for individuals with environmental or personal circumstances that increase the probability of either contracting the disease they are meant to prevent or have more severe forms of the disease if they were to get it, or because they have an underlying condition that could get exacerbated or decompensated if they were infected with the disease.
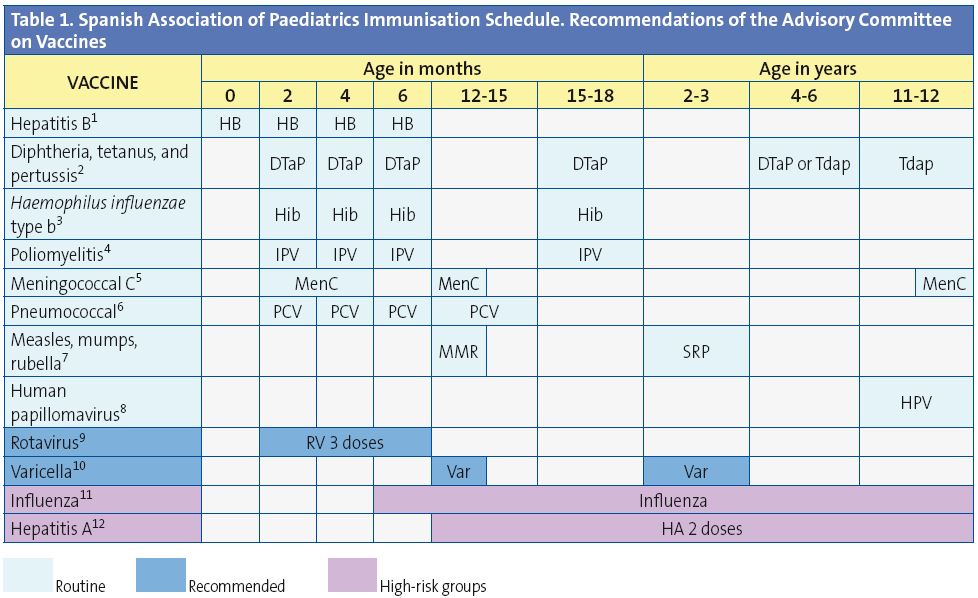
(1) Hepatitis B vaccine (HB). 3 doses following one of 3 equivalent schedules: 0, 1, 6 months, or 0, 2, 6 months, or 2, 4, 6 months, all suitable for children of seronegative mothers (HBsAg-), with the first 2 also indicated for hepatitis B virus carrier mothers (HBsAg+). The latter group of newborns (HBsAg+ mothers) will be given the first dose of the vaccine and 0.5 ml of hepatitis B immune globulin within the first 12 hours of life, the 2nd dose of the vaccine at 1 or 2 months of age, and the 3rd dose 6 months after birth. If the serostatus of the mother were unknown, the 1st dose of the vaccine should be administered within the first 12 hours of life and the mother’s serostatus assessed, and should the mother test positive, 0.5 ml of hepatitis B immune globulin should be administered in the first week of life (preferably within 72 hours after birth). The administration of 4 doses of the HB vaccine is acceptable in children vaccinated with the 1st dose of the monovalent preparation at birth, followed by 3 doses of the hexavalent vaccine at 2, 4, and 6 months of age. Unvaccinated older children and adolescents of any age will be given 3 doses on a 0, 1, 6 months schedule.
(2) Diphtheria, tetanus, and acellular pertussis (DTaP/Tdap). 6 doses: primary vaccination with 3 doses of the DTaP vaccine; booster at 15-18 months of age (4th dose) with the DTaP vaccine; at 4-6 years (5th dose) with the DTaP or with the reduced-antigen content tetanus and diphtheria vaccine (Tdap) and at 11-12 years (6th dose) with the Tdap.
(3) Haemophilus influenzae type b conjugate vaccine (Hib). 4 doses: primary vaccination at 2, 4, 6 months and booster at 15-18 months (4th dose).
(4) Inactivated poliovirus vaccine (IPV). 4 doses: primary vaccination with 3 doses, and a booster at 15-18 months (4th dose).
(5) Meningococcal C conjugate vaccine (MenC). 3 or 4 doses of the monovalent conjugate vaccine (with a 1 or 2+1+1 scheme), on the following schedule: 1 or 2 doses between 2 and 11 months of age, another dose at 12 months of age, and a final dose at 12 years. The vaccination course for infants 2 to 4 months of age, of 1 or 2 doses, will depend on which vaccine preparation is used.
(6) Pneumococcal conjugate vaccine (PCV). 4 doses: the first 3 at 2, 4, 6 months of age with a booster some time between 12 and 15 months of age (4th dose).
(7) Measles, mumps, and rubella vaccine (MMR). 2 doses of the measles-mumps-rubella vaccine. The first dose at 12 months of age, and the second at 2-3 years, preferably at 2.
(8) Human papillomavirus vaccine (HPV). Only for girls.2 or3 doses between 11 and 12 years of age. The tetravalent should be given on a 0, 2, 6 month schedule and the bivalent vaccine as a 2-dose series (at 0 and 6 months) for girls between 9 and 14 years of age, and a 3-dose series (at 0, 1, 6 months) for patients 15 years of age or older.
(9) Rotavirus vaccine (RV). 3 doses of the rotavirus vaccine: at 2, 4, 6 months or 2, 3, 4 months. The course must be started between 6 and 12 weeks after birth and must be completed by 32 weeks of age.
(10) Varicella vaccine (Var). 2 doses: the 1st at 12 months (administration between 12 and 15 months is acceptable) and the 2nd at 2-3 years of age, preferably at 2 years. Susceptible patients outside the aforementioned age range should be immunised with 2 doses at least one month apart.
(11) Seasonal flu (Influenza). At-risk patients and their household members (older than six months of age) should be immunised yearly. 1 dose in individuals over 9 years of age; individuals between 6 months and 9 years of age will be given 2 doses 1 month apart the first time they get vaccinated, and if the risk factor persists, they will be immunised yearly with a single dose.
(12) Hepatitis A vaccine (HA). 2 doses, at a 6-12 month interval, starting at 12 months of age. Vaccination of patients for whom it is indicated due to international travel to countries with a high to moderate endemicity, or who belong to high-risk groups.
Immunisation schedules must be dynamic and adapt to the epidemiological shifts that may emerge. Considering the latest shifts in disease epidemiology, new publications on the efficacy, effectiveness, and safety of the different vaccines, and recent changes to the summary of product characteristics of some vaccine preparations, the CAV-AEP wishes to underscore the following recommendations:
- Hepatitis B: recommended for the first year of life, to be administered in 3 to 4 doses of the monovalent or hexavalent commercial preparation. Older children who have not been immunised will be given a 3-dose series of the monovalent vaccine on a schedule of 0, 1, and 6 months.
- Dyptheria-tetanus-pertussis-poliomyelitis-Haemophilus influenzae type b: we recommend primary vaccination with the DTaP-IPV-Hib-HB at 2, 4, and 6 months of age. It is also acceptable to administer the hexavalent or pentavalent vaccines in combination with the monovalent hepatitis B vaccine. The option of administering the first dose earlier at 6 weeks of age can be evaluated. A booster dose of DTaP-IPV-Hib must be administered at 15-18 months, another at 4-6 years of age of the DTaP or Tdap, and a booster dose of the Tdap at 11-12 years of age. Immunisation of pregnant women with Tdap is recommended after 27 weeks of gestation, as well as immunisation of the household members that will have close contact with the newborn (the cocooning strategy), and especially of unvaccinated postpartum mothers.
- Meningococcal group C: the immunisation schedule for the meningococcal C vaccine has changed from a 2+1 scheme to a 3- to 4-dose series of the monovalent conjugate vaccine (with a 1 or a 2+1+1 scheme) with the following course: 1 or 2 doses between 2 and 11 months of age, another dose at 12 months, and a final dose at 12 years. The course for infants 2 to 4 months of age, of 1 or 2 doses, will depend on the vaccine preparation to be used.
- Pneumococcal: routine immunisation of all children younger than five years of age is recommended as the best means to prevent pneumococcal disease in childhood, with the conjugated 13-valent pneumococcal vaccine (VNC13) offering the best coverage against the pneumococcal serotypes circulating in Spain. We also want to underscore the need to vaccinate all children between 6 and 7 years of age who are immunocompromised or otherwise at-risk (Table 2).
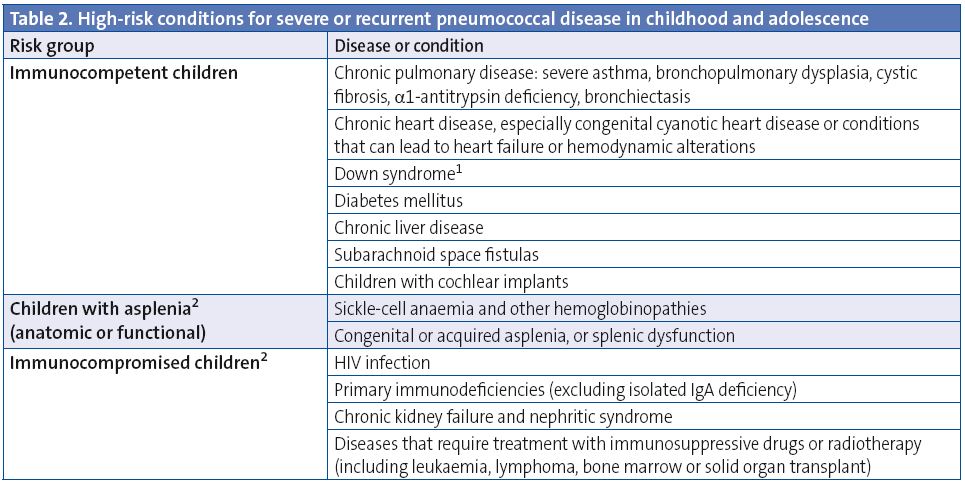
- In case of a documented immunodeficiency with high risk for IPD, follow the recommendation for immunocompromised children.
- High-risk patients: follow the specific recommendations for immunisation against pneumococcal disease (see the text).
- Measles, mumps, and rubella (MMR): the default course of vaccination is maintained, with the administration of 2 doses, the first one recommended at 12 months of age, and the second one at 2-3 years, preferably at 2. Whenever necessary, a minimum interval of 4 weeks between both doses will result in a correct immunisation. We would also like to underscore the need to reach and maintain high vaccination coverage rates to achieve herd immunity and facilitate the eradication of the diseases prevented by this vaccine.
- Human papillomavirus: routine immunisation of all girls 11-12 years of age is recommended as a means to prevent cervical cancer and precancerous cervical lesions in women. It can be performed with either the bivalent or the tetravalent preparation. In addition to the customary 3-dose course, a 2-dose course has been recently approved (on a 0 and 6 month schedule) for girls 9 to 14 years old with the bivalent preparation. All healthcare professionals must make a big effort to increase the current vaccine coverage rates.
- Rotavirus: taking into account its morbidity and high burden of disease, vaccination against rotavirus is recommended for all infants, using the pentavalent preparation now available in Spain. We recommend the administration of 3 doses. The first one is to be given between 6 and 12 weeks of age. The minimum interval between doses is of 4 weeks. All 3 doses must be administered before 32 weeks of age. They can be given at the same time as other vaccines in the schedule.
- Varicella: universal vaccination against varicella started in the second year of life is an effective strategy, and we request that the two existing vaccines are made immediately available to the public in Spain, upholding the right to have it prescribed and the right of healthy children to be immunised. We recommend that all children are vaccinated against varicella with two doses: a first dose at 12 months of age, and a second one at 2-3 years, preferably at 2.
- Influenza: yearly immunisation is recommended in children and adolescents in: a) high-risk groups: children 6 months of age or older and adolescents who have specific underlying circumstances or conditions; b) healthy children six months of age and older and healthy adolescents who live with high-risk patients; and c) adults who come in contact with children and adolescents who belong to the high-risk group. It is of particular importance that all health professionals get vaccinated against the seasonal flu every year.
- Hepatitis A: immunisation is recommended for certain high-risk situations, administering two doses at least 6 months apart. The physician should evaluate its administration in children older than 12 months who attend a nursery.
The vaccine against group B meningococcal disease, which was recently approved, brings new hope for the prevention of this disease. While we wait for upcoming local and international studies, for the time being we recommend its use to control disease outbreaks, and urge that it is made available for sale in pharmacies.
More information on all of the aspects covered here can be found in the paper published in the January 2014 issue of the journal Anales de Pediatría1 and in the CAV-AEP website (www.vacunasaep.org).
POSITION OF THE CAV-AEP ON THE 2014 UNIFIED IMMUNISATION SCHEDULE OF THE MINISTRY OF HEALTH
At present, there are no epidemiological differences among the different Autonomous Communities (the regional administrations into which Spain is divided) for vaccine-preventable diseases, with the possible exception of hepatitis A in Ceuta and Melilla, to justify the existence of different immunisation schedules2. The CAV-AEP believes that all healthcare and policy agents involved in making decisions about the design and funding of the immunisation schedule for children residing in Spain have to make a concerted effort, and continues to offer its cooperation to achieve this sensible goal. In this regard, we have expressed our opposition to the new unified schedule proposed by the Consejo Interterritorial (Interterritorial Council of the Spanish National Health Service) in March 2013, which was later modified in December 20133 , because we believe it would exacerbate the problems involved in the implementation of a unified schedule in the various Autonomous Communities, and because it is insufficient to achieve the best and greatest possible protection for all Spanish children, given that it does not consider the addition of new routine immunisations even as a future option once economic circumstances improve.
We think that we ought to expect the Ministry of Health and the different Autonomous Communities to make a collective economic effort allowing the funding of a comprehensive routine schedule rather than one offering minimum coverage to Spanish children. We recommend reading the full arguments that support the position of this Committee on the implementation of the unified schedule of the Ministry of Health, recently published in the January issue of the journal Anales de Pediatría4.
CATCH-UP IMMUNISATION SCHEDULES AND COURSES FOR CHILDREN AND ADOLESCENTS WITH INCOMPLETE IMMUNISATION
In many occasions it is necessary to immunise children who have not been given any vaccines, or who have either not followed a schedule regularly, have started it late, have stopped getting immunisations, or have been vaccinated in countries of origin according to schedules that differ from the one in Spain. All these children must be vaccinated to comply with the domestic immunisation schedule. We have developed tables to guide in the design of catch-up vaccination courses in children and adolescents with incomplete immunisation (Tables 3 to 5) which differ in some points from the 2013 tables.
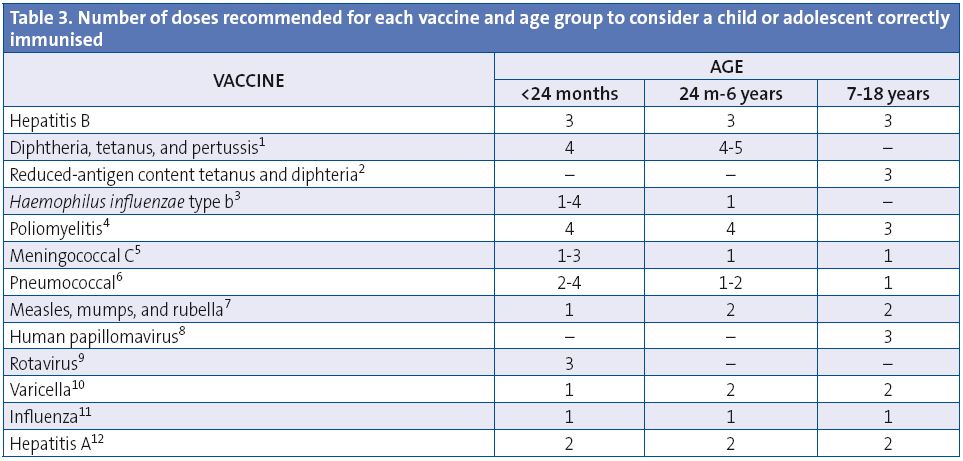
These table shows the number of necessary doses, for each age, for children and adolescents with incomplete immunisation, or who are starting immunisations late. A vaccination course needs not be restarted if doses have been previously administered, but should be completed regardless of whether the maximum interval has elapsed since the last dose. Adverse reactions must be notified to the healthcare authorities.
(1) Diphtheria, tetanus, and acellular pertussis vaccine (DTaP). The 5th dose of DTaP or Tdap is not needed if the 4th dose of DTaP was give at 4 years of age or later. The DTaP vaccine can be used until age 6 years. The Tdap, with reduced diphtheria and pertussis antigen content, is approved for use in patients 4 years of age and older.
(2) Reduced-antigen content tetanus and diphtheria vaccine (Td). Children 7 years of age or older should be given the reduced-antigen content tetanus and diphtheria vaccine. Once primary vaccination is completed, it is recommended that the Tdap vaccine is used for booster doses.
(3) Haemophilus influenzae type b conjugate vaccine (Hib). The number of doses depends on the age at start of immunisation: 4 for children younger than 6 months, 3 for children between 7-11 months; 2 for children between 12-14 months; and one 1 for children 15 months to 5 years.
(4) Inactivated poliovirus vaccine (IPV). A 4th dose is only required if the 3rd dose was administered before 4 years of age.
(5) Meningococcal C conjugate vaccine (MenC). Depends on age: 1 or 2 doses, depending on the vaccine preparation, in children younger than 12 months, with 1 booster in the 2nd year of life, and another booster during adolescence at 12 years of age; 1 dose in children vaccinated at age over 12 months, with a one-time booster in adolescence.
(6) Pneumococcal conjugate vaccine (PCV). Number of doses according to the age at start of immunisation: 4 in children who were younger than 6 months, 3 in children who were 7-11 months; 2 for children 12-23 months; for children ages 24 months to 5 years: 1 dose of Prevenar 13® (1 or 2 in high-risk groups, refer to text) and 2 of Synflorix®; between 6 and 17 years, 1 dose of Prevenar 13® in high-risk groups (see text). Synflorix® is approved for use up to 5 years of age, and Prevenar 13® is approved through adulthood, with no age limits.
(7) Measles, mumps, and rubella vaccine (MMR). 2nd dose can be given starting at 2-3 years of age.
(8) Human papillomavirus vaccine (HPV). Only for girls. 2 or 3 doses between 11 and 12 years of age. The Consejo Interterritorial recommends its administration at 14 years of age.
(9) Rotavirus vaccine (RV). 3 doses of the rotavirus vaccine (RotaTeqÒ). The course can start between 6 and 12 weeks of age and must be completed before 32 weeks of age.
(10) Varicella vaccine (Var). 2nd dose starting at 2-3 years of age.
(11) Seasonal flu (Influenza). 1 annual dose of the inactivated influenza vaccine starting at 6 months of age. The children younger than 9 years will be given 2 doses the first time they are vaccinated, at least one month apart.
(12) Hepatitis A vaccine (HA). 2 doses, separated by at least 6-12 months, starting at 12 months of age.
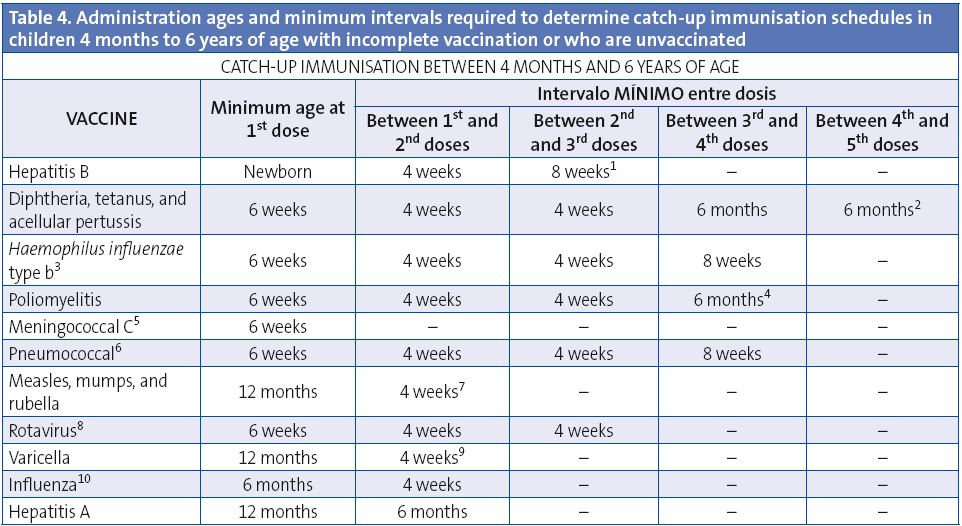
This table shows the minimum intervals between doses for children with incomplete immunisation, or who are starting immunisations late. A vaccination course needs not be restarted if doses have been previously administered, but should be completed regardless of whether the maximum interval has elapsed since the last dose. Adverse reactions must be notified to the healthcare authorities.
(1) Hepatitis B vaccine (HB). The 3rd dose will be administered at least 4 months after the 1st one, and never before 6 months of age. If 1 dose of the monovalent was been administered after birth, it is also acceptable to administer 3 additional doses with the hexavalent preparation; the last dose should always be given at 6 months of age or later.
(2) Diphtheria, tetanus, and acellular pertussis vaccine (DTaP/Tdap). The minimum interval between the 3rd and 4th doses of the DTaP is 6 months, but if the 4th dose is given after 4 months, the vaccination will be considered valid. The 5th dose of the DTaP or Tdap is not needed if the 4th dose of the DTaP was administered at 4 years of age or later.
(3) Haemophilus influenzae type b conjugate vaccine (Hib). Every dose given before 12 months of age will be administered at least 4 weeks apart. If the 1st dose of the series is given between 12 and 14 months of age, the 2 doses should be 8 weeks apart. If the 1st dose is given at 15 months of age or later, only one dose is needed. The 4th dose should only be administered if 3 doses have been given in the first 12 months of life.
(4) Inactivated poliovirus vaccine (IPV). A 4th dose needs only be given if the 3rd dose was administered before 4 years of age, in which case these doses should be 6 months apart.
(5) Meningococcal C conjugate vaccine (MenC). 1 or 2 doses should be given in the first year of life (at 2 and 4 months or at 4 months) depending on the vaccine preparation. From at 12 months of age, 1 dose should be given in the second year of life, and 1 dose during adolescence at 12 years of age.
(6) Pneumococcal conjugate vaccine (PCV). All doses administered before 12 months of age must be given at least 4 weeks apart. If the vaccine is administered between 12 and 24 months, the 2 doses will be 8 weeks apart. If the 1st dose is given at an age greater than 24 months, only 1 dose of Prevenar 13® is needed, or 2 of Synflorix ® given 8 weeks apart, except in high-risk groups, which require 2 doses of either preparation. Children older than 5 years do not require immunisation, except for those in high-risk groups, who must be given 1 dose of Prevenar 13®. The 4th dose will only be administered if 3 doses were given in the first year. The 23-valent polysaccharide vaccine is indicated in children older than 2 years with conditions that increase the risk of pneumococcal infection; the interval that must elapse since the last dose of pneumococcal conjugate vaccine is of 8 weeks. Synflorix® is approved for use until 5 years of age, and Prevenar 13® through adulthood, with no age limits.
(7) Measles, mumps, rubella vaccine (MMR). Administer the 2nd dose at 2-3 years of age, preferably at 2. From the age of 12 months, a child is considered correctly immunised if 2 doses are given at least 4 weeks apart.
(8) Rotavirus vaccine (RV). 3 doses of the pentavalent preparation (RotaTeq®), the last one to be given prior to 32 weeks of age.
(9) Varicella vaccine (Var). Administer the 2nd dose at 2-3 years, preferably at 2 years along with the MMR vaccine (either the same day, or at least 1 month apart). The minimum interval between the 2 doses of the varicella vaccine is of 4 weeks, although in children younger than 13 years an interval of 6 to 12 weeks is recommended.
(10) Seasonal flu (Influenza). Only children younger than 9 years who are being vaccinated for the first time will be given 2 doses, at least one month apart.
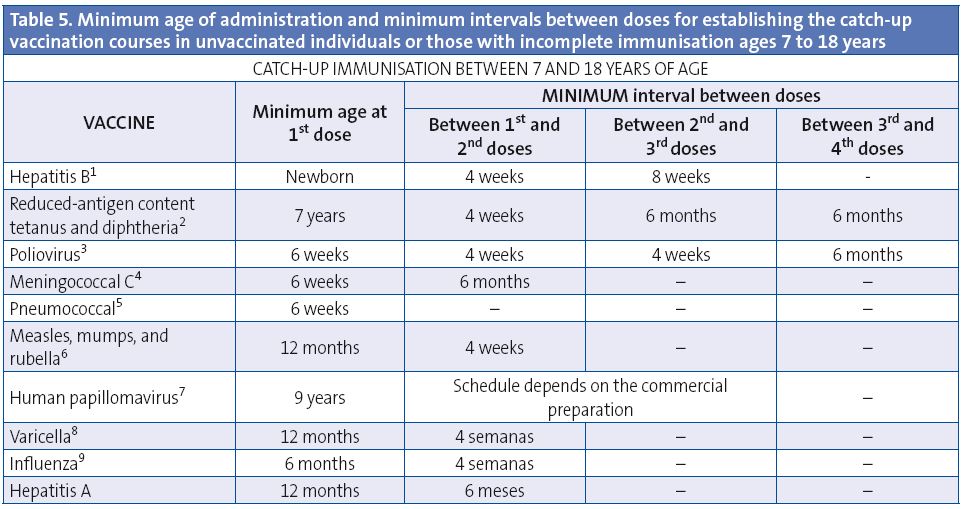
This table shows the minimum intervals between doses for children and adolescents with incomplete immunisation or who are starting immunisations late. A vaccination course needs not be restarted if doses have been previously administered, but should be completed regardless of whether the maximum interval has elapsed since the last dose. Adverse reactions must be notified to the healthcare authorities.
(1) Hepatitis B vaccine (HB). In unvaccinated children over 7 years of age, 3 doses at 0, 1, 6 months. The 3rd dose will be administered at least 4 months after the 1st dose.
(2) Reduced-antigen content tetanus and diphtheria vaccine (Td). Starting from 7 years of age, the reduced-antigen content tetanus and diphtheria vaccine (Td) should be used. Once primary vaccination is completed, we recommend using the reduced-antigen content tetanus-diphtheria-acellular pertussis vaccine (Tdap) for booster doses. Those vaccinated with 1 dose of DTP before 12 months of age will be given, when resuming immunisation past 7 years of age, 2 additional doses of Td to complete primary vaccination and 1 booster dose of Tdap. Those vaccinated with 1 dose of DTP or Td after 12 months of age, when resuming immunisation after 7 years of age, will complete primary vaccination with 2 doses of Td six months apart. For adults to be considered completely immunised against tetanus, they must have received at least 5 doses of vaccine with tetanus toxoid in their lifetime, so following primary vaccination with 3 doses, two booster doses must be administered, preferably 10 years apart, although the minimum interval between the two doses is of 1 year, and one of them should be a dose of Tdap.
(3) Inactivated poliovirus vaccine (IPV). In unvaccinated patients older than 7 years, 3 doses. If the 3rd dose was given before 4 years of age, administration of a 4th dose is recommended at least 6 months after the 3rd one.
(4) Meningococcal C conjugate vaccine (MenC). In unvaccinated patients older than 7 years and younger than 10, administer 1 dose and another one after 10 years of age, with a minimum interval of 6 months between both doses. If the patient is older than 10 years, only 1 dose is necessary.
(5) Pneumococcal conjugate vaccine. Prevenar 13® is approved through adulthood, with no age limits. All unvaccinated individuals in high-risk groups will be given 1 dose of this vaccine and 1 dose of the 23-valent polysaccharide vaccine 8 weeks later. If they had already received one dose of the 23-valent vaccine, they can be given 1 dose of Prevenar 13® at least 8 weeks apart from the 23-valent vaccine dose. High-risk groups will be given a 2nd (and last) dose of the 23-valent vaccine 5 years after the 1st dose.
(6) Measles, mumps, rubella vaccine (MMR). In unvaccinated children older than 7 years, 2 doses. If the child was previously immunised with 1 dose of monovalent measles vaccine, administer 2 doses of MMR. If the child was previously immunised with 1 dose of MMR, administered a 2nd dose.
(7) Human papillomavirus vaccine (HPV). Only for girls.The minimum age for administering the 1st dose is 9 years. Whenever possible, it is best to follow the immunisation schedule specified for the corresponding commercial preparation: Gardasil® 0, 2, 6 months; Cervarix® a 2-dose series (at 0 and 6 months) in girls 9 to 14 years, and a 3-dose series (at 0, 1, 6 months) for patients 15 years or older. Gardasil® recommends that the 2nd dose be administered at least 1 month after 1st dose, and the 3rd dose at least 3 months after the 2nd dose; the 3rd dose should be given at least 6 months after the first one, but immunisation will be considered correct if it has been at least 4 months since the 1st dose. All 3 doses must be administered in the span of 1 year. Cervarix® recommends that the 2nd dose be administered between 1 and 2.5 months after the 1st dose, and the 3rd dose between 5 and 12 months after the 1st dose.
(8) Varicella vaccine (Var). 2 doses at least 4 weeks apart in previously unvaccinated patients. In children younger than 13 years an interval of 6-12 weeks is recommended between the two doses, and in those older than 13, an interval of 4 to 8 weeks.
(9) Seasonal flu (Influenza). Two doses, at least 4 weeks apart, will be given only to children younger than nine years the first season they are vaccinated against influenza.
CONFLICTO DE INTERESES
These are outlined in detail in the original publication1.
ABBREVIATIONS: CAV-AEP: Advisory Committee on Vaccines of the Spanish Association of Pediatrics
BIBLIOGRAPHY
- Moreno-Pérez D, Álvarez García FJ, Arístegui Fernández J, Cilleruelo Ortega MJ, Corretger Rauet JM, García Sánchez N, et al.; en representación del Comité Asesor de Vacunas de la Asociación Española de Pediatría. Calendario de vacunaciones de la Asociación Española de Pediatría: recomendaciones 2014. An Pediatr (Barc). 2014;80:55.e1-55.e37.
- Calendarios de vacunación de las ciudades y comunidades autónomas españolas. Web de la Asociación Española de Pediatría de Atención Primaria [on line] [consulted on 18/01/2014]. Available in www.aepap.org/vacunas/calendarios-espanoles
- Consejo Interterritorial del Sistema Nacional de Salud. Calendario común de vacunación infantil; enero de 2014 [on line] [consulted on 18/01/2014]. Available in www.msssi.gob.es/ciudadanos/proteccionSalud/vacunaciones/docs/CalendarioVacunacion2014.pdf
- Arístegui Fernández J, Moreno-Pérez D. El calendario de vacunación común de mínimos para España: posicionamiento del CAV-AEP. An Pediatr (Barc). 2014;80:1-5.





