Vol. 14 - Num. 55
Consensus document
Consensus document on the aetiology, diagnosis and treatment of acute otitis media
F del Castillo Martína, Fernando Baquero Artigaob, T de la Calle Cabrerac, MV López Roblesd, J Ruiz Canelae, Santiago Alfayate Miguélezf, F Moraga Llopb, Cristina Calvo Reyb
aSociedad Española de Infectología Pediátrica (SEIP).
bSociedad Española de Infectología Pediátrica (SEIP). España.
cSociedad Española de Pediatría Extrahospitalaria y Atención Primaria (SEPEAP).
dSociedad Española de Urgencias Pediátricas (SEUP).
eAsociación Española de Pediatría de Atención Primaria (AEPap).
fSección de Infectología Pediátrica. Hospital Clínico Universitario Virgen de la Arrixaca. Murcia. España.
Reference of this article: del Castillo Martín F, Baquero Artigao F, de la Calle Cabrera T, López Robles MV, Ruiz Canela J, Alfayate Miguélez S, et al. Consensus document on the aetiology, diagnosis and treatment of acute otitis media. Rev Pediatr Aten Primaria. 2012;14:195-205.
Published in Internet: 06-08-2012 - Visits: 88514
Abstract
We present the consensus document on acute otitis media (AOM) written by the Spanish Society of Pediatric Infectology (SEIP), the Spanish Society of Outpatient and Primary Care Pediatrics (SEPEAP), the Spanish Society of Pediatric Emergency Care (SEUP) and the Spanish Association of Primary Care Pediatrics (AEPAP).
The document analyses the etiology of the disease and the possible shifts in it following the introduction of the 7-valent, 10-valent, and 13-valent pneumococcal vaccines. The document proposes diagnosing AOM as confirmed or probable. The AOM diagnosis is considered confirmed if three criteria are met: acute onset, signs of fluid in the middle ear (or otorrhea), and symptoms of inflammation, such as otalgia or marked erythema in the middle ear, and considered probable when only two of these criteria are met. The proposed first choice for antibiotic treatment is 80 mg/kg/day of amoxicillin administered orally in doses at eight hour intervals. Treatment with amoxicillin-clavulanic acid in doses of 80 mg/kg/day are indicated in children younger than six months, in infants with a severe presentation (fever >39 ºC or acute pain), when there is a family history of AOM sequelae, or in cases of amoxicillin treatment failure.
Keywords
● Acute otitis media ● Amoxicillin ● Etiology ● SymptomatologyNote:
Article publihed simultaneously with Anales de Pediatría: del Castillo Martín F, Baquero Artigao F, de la Calle Cabrera T, López Robles Mv, Ruiz Canela J, Alfayate Miguélez S, et al. Documento de consenso sobre etiología, diagnóstico y tratamiento de la otitis media aguda. An Pediatr (Barc). 2012;77 (11):345.e1-345.e8 [on line]. Available in: http://dx.doi.org/10.1016/j.anpedi.2012.05.026
INTRODUCTION
Acute otitis media (AOM) is one of the most frequently occurring childhood diseases, and the main cause for prescribing antibiotics to children in developed countries1. Furthermore, some studies2 have found that this condition is overdiagosed in children, resulting in the excessive use of antibiotic treatment, with the subsequent incidence of side effects and increase of bacterial resistance. This is the reason why in the past few years numerous papers and clinical guidelines have been devoted to the accurate diagnosis and appropriate treatment of acute otitis media in children.
As is customary in other consensus documents, we will rate the strength of the recommendation (A: good evidence; B: moderate evidence; C: poor evidence) and the quality of the scientific evidence (I: randomised controlled clinical trials; II: well-designed clinical trials without randomisation; III: opinions of authorities based on clinical experience or descriptive studies) supporting the proposed measures, following the grading system established by the Infectious Disease Society of America.
ETIOLOGY OF ACUTE OTITIS MEDIA
Microbiology and influence of immunisations
The bacterial agents that caused AOM in our environment prior to the introduction of the pneumococcal vaccine were3 Streptococcus pneumoniae (S. pneumoniae) (35%), non-typeable Haemophilus influenzae (H. influenzae) (25%), Streptococcus pyogenes (3-5%), Staphylococcus aureus (1-3%), and Moraxella catarrhalis (M. catarrhalis) (1%). Other microorganisms that cause AOM less frequently in healthy children are Escherichia coli, Pseudomonas aeruginosa, and anaerobes, and in very rare cases Mycoplasma pneumoniae, Chlamydia, and some fungi. For reasons that are yet to be understood, between 20 and 30% of the cultures of middle ear exudates do not grow.
Nevertheless, it is well known that conjugate pneumococcal vaccines decrease the nasopharyngeal colonisation rates of the included serotypes, while facilitating an increase in the colonisation rate by non-vaccine serotypes4-7, which can make the frequencies noted above shift. A recent analysis carried out by the National Centre of Microbiology of Majadahonda on pneumococcal isolates from otic exudates between years 2001 and 20098 showed a significant decrease in the vaccine serotypes (from 62.9% in 2001 to 10.6% in 2009) and an increase in non-vaccine serotypes, especially in types 3, 6A, and 19A (the latter showed the highest increase, from 9.5 to 35.5%). Globally, in the last decade, 68% of AOM cases were caused by serotypes not included in the heptavalent vaccine, and 43% were caused by serotype 19A9.
The eradication of vaccine serotypes from the nasopharynx by the heptavalent conjugate vaccine opens up an ecological niche that is occupied not only by non-vaccine serotypes, but also by many other biological competitors, especially H. influenzae5. There has been a proven increase in otitis cases caused by this microorganism in populations with high rates of pneumococcal immunisation10, and some studies show it is the bacterium most often causing AOM, even ahead of Pneumococcus (56-57% versus 31%). It is not known whether this is a generalised and lasting phenomenon.
Two new conjugate pneumococcal vaccines have been developed very recently which may have an influence on these data: the 10-valent vaccine (Synflorix®), which adds serotypes 1, 5, and 7F to those of the heptavalent preparation, and the 13-valent vaccine (Prevenar-13®), which further includes 3, 6A, and 19A. Both have been approved for the prevention of invasive pneumococcal disease and AOM caused by pneumococcus in children six weeks through five years of age.
One of the most interesting characteristics of the 10-valent vaccine is that the pneumococcal serotypes are conjugated to protein D, a lipoprotein found in most H. influenzae strains, thus providing protection against the two main pathogens that cause AOM. In a randomised and double-blind clinical trial, the vaccine showed a 33.6% efficacy against any type of AOM, a 57.6% efficacy against AOM produced by vaccine serotypes, and a 35.6% efficacy in preventing AOM caused by H. influenzae11.
There are no studies on the efficacy of the 13-valent vaccine in children with AOM. If we compare the two new vaccines, the 13-valent has no protective effect against H. influenzae, but it includes emerging pneumococcus serotypes that cause AOM, especially 19A. A recent study has proven a reduction in the rate of nasopharyngeal colonisation by serotype 19A in children with AOM that had been immunised with the 13-valent vaccine, compared to those children immunised with the heptavalent preparation12.
Another significant problem concerning AOM pathogens is their varying behaviour in the middle ear cavity. The persistence of ear discharge in children that have not been treated with antibiotics after 2-5 days is higher than 80% for S. pneumoniae, about 50% for H. influenzae and about 21% for M. catarrhalis13. This means that S. pneumoniae is the main pathogen causing AOM and the bacterium that shows the lowest rate of spontaneous clearance from the middle ear.
There is more controversy when it comes to the role played by viruses in the etiology of AOM. Starting in the 1970s with the publication of the work of Klein and Teele14, there has been agreement that a viral infection of the respiratory tracts can facilitate the development of otitis media, but that it does not cause it directly. However, later studies have found that viruses were the sole pathogen in 3 to 13% of AOM isolates15. Nevertheless, there is no consensus that viruses have a role in the etiology of AOM, especially since their ability to replicate in the middle ear has yet to be demonstrated.
Antibiotic resistance
According to the data of the latest susceptibility study for antimicrobials used in the Spanish population (SAUCE), the overall resistance of pneumococcus to penicillin is 23%, with 0.9% of strains showing high-level resistance (MIC ≥ 2 ug/ml)16. The resistance rates are higher in children (27%) and in middle ear isolates (31%). These are the lowest figures in the last decade, and in the past few years we have been observing a significant decrease in the frequency of resistant isolates, a decrease that has been more marked in the pediatric population. There are probably several causes for this, but pneumococcal immunisation is considered a chief one among them, since it has managed to decrease the infection rates of the most resistant serotypes.
According to data from the National Centre for Microbiology8, the rates of resistance to penicillin (51%) and to erythromycin (45%) in pneumococcal AOM have remained relatively stable in the past ten years. Furthermore, there has been evidence of a significant increase in amoxicillin resistance (from 8% in 2001 to 24% in 2009), parallel to the increase in the resistance to this antibiotic in serotype 19A (from 0% in 2001 to 38% in 2009). At present, 19A is the most frequently found antibiotic-resistant serotype; in AOM and mastoiditis isolates, the resistance rates were 60% for penicillin, 76% for erythromycin, and 36% for cefotaxime17, and in recurrent or persistent AOM, the rates were 78% for amoxicillin, 88% for erythromycin, and 33% for cefotaxime.
As for H. influenzae, several studies done in the United States have shown an increased proportion of beta-lactamase producing organisms in AOM isolates from children vaccinated against pneumococcus9,10, although this has not been seen in our country, where only a small percentage of H. influenzae isolates in children with AOM were positive for these enzymes. In the last SAUCE study, 16% of the isolated organisms produced beta-lactamases, a figure that has dropped significantly in the last few years16. This decrease in beta-lactamase production in H. influenzae strains may be related to the lower consumption of antibiotics in the population18.
DIAGNOSIS OF ACUTE OTITIS MEDIA
The diagnosis of AOM in children is based on clinical examinations and otoscopic exams. The difficulty diagnosis presents is due to several factors, and mainly to the non-specificity of symptoms (which increases the younger the child) and the difficulty of making the otoscopic exam (narrow and winding ear canal, not very cooperative patient, etc.). In order to improve the accuracy of the diagnosis, we must try to base it on parameters as objective as possible, defining a series of clinical and otoscopic criteria.
Definition of clinical forms
To better approach the diagnosis (and then the management) of AOM, we must define otitis media as the presence of exudate in the middle ear cavity19. Depending on the presenting history and the characteristics of the exudate, it can be distinguished into:
- AOM: symptomatic presence of exudate in the middle ear (usually mucopurulent). This is the clinical picture we will be referring to most of the time, of which we can distinguish different presentations:
- Sporadic AOM: isolated episodes.
- Repeated AOM: repeated episodes, which is in turn classified into:
- Persistent AOM: AOM symptoms recur in the first seven days following treatment completion (it is considered to be the same episode).
- Relapsing AOM (true relapse): return of the symptoms after seven days post-treatment (it is considered a separate episode).
- Recurrent AOM: tendency to get AOM when there is an upper respiratory tract infection. It is defined as at least three episodes within six months or at least four within one year.
- Otitis media with effusion or nonsevere otitis media (wrongly called serous otitis media): evidence of fluid in the middle ear space without associated symptoms (except for transmission hypoacusia). It usually presents following AOM, but resolves spontaneously in 90% of the cases. If it persists for longer than three months, it is defined as chronic otitis media with effusion.
- Chronic otitis media with effusion: infection of the middle ear lasting more than three months.
Clinical picture
Otalgia is the most specific clinical feature of AOM20, but it is hard to assess pain in young children, so we could consider irritability or intense crying (especially starting at night after a few hours of sleep) as “otalgia equivalents”21. Acute (purulent) otorrhea is highly suggestive of AOM. Although catarrhal symptoms are observed in 70-90% of AOM cases, they have little value for differential diagnosis. There are usually other non-specific symptoms, such as fever, vomiting, and refusal of food, but these clinical data in themselves do not help us differentiate between AOM and an upper respiratory tract infection in children younger than three years (in whom otalgia is difficult to assess)22. Although it is more characteristic of external otitis, in infants, whose ear canal is cartilaginous, painful swallowing is also found usually in AOM. The development of conjunctivitis along with AOM has been associated traditionally with infection by H. influenzae.
Examination
- General: assessment for signs of baceraemia-sepsis, such as weakness, poor general health status, exhaustion, and alterations in cutaneous vascular perfusion. Meningeal and neurological signs should also be assessed, since there is the possibility of intracranial complications.
- Regional: AOM is usually accompanied by inflammatory processes in the upper respiratory tracts, and it may produce regional complications, so the nasal passages, the oropharynx and the cervical and mastoid regions must be examined.
- Local (otoscopy): after assessing the “low specificity” clinical symptoms, the findings of the otoscopy are key in making an accurate diagnosis of AOM23. Thus, examining the eardrum is of utmost importance, if necessary removing the cerumen or secretions that may obstruct the outer ear canal. The otoscopy may reveal:
The use of the pneumatic otoscope (which is scarcely available in Spanish pedriatic offices, but highly recommended by American guidelines) can assess for impaired mobility of the membrane, which provides “objective” evidence that there is exudate in the middle ear, and thus can increase the accuracy of the diagnosis.
Diagnostic tests
Although they are not usually required for diagnosing AOM, in some cases diagnostic tests must be done if there is a likelihood that the patient will develop complications (sepsis, meningitis, mastoiditis...): blood tests (complete blood count, blood differential and acute phase reactants), blood culture, lumbar puncture, or computerised tomography scan of the skull and the temporal bone.
Microbiological tests are not usually needed, but a culture and antibiogram of the spontaneous otorrhea (if it occurs) are recommended. In select cases, it may be convenient to take a fluid sample by means of a myringotomy or a tympanocentesis, for instance in cases of AOM that are not responding to treatment, for instance, as well as recurrent AOM, or in patients that have developed complications21.
Diagnostic criteria for acute otitis media
According to the clinical practice guideline on otitis media presented by the American Academy of Pediatrics/American Academy of Family Physicians in 2004, the diagnosis must be based on three criteria25:
- Acute onset of symptoms.
- Otoscopic signs of middle ear effusion: bulging, pathological pneumatoscopy, or otorrhea.
- Signs or symptoms of inflammation (otalgia or intense tympanic membrane erythema).
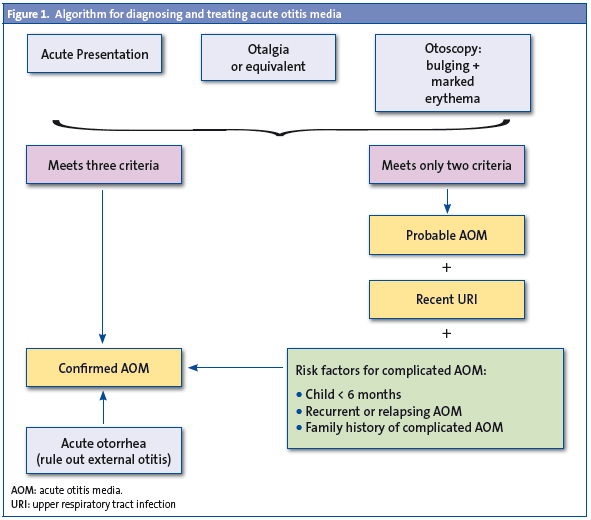
Nevertheless, the strict application of these criteria may leave cases of AOM undiagnosed, so in 2007 the consensus document on AOM of the Spanish Association of Pediatrics and Otorhinolaryngology19 specified that the diagnosis be “confirmed AOM” when all three criteria are met, but if there is only evidence of otalgia and it is not possible to perform an otoscopy (if, for example, there is a manifest technical difficulty or cerumen that cannot be extracted) or, on the contrary, the otoscopy is very significant but the otalgia is not clear or confirmed (due to the age of the child, uncertainty of the family members, etc), the consensus proposes that the diagnosis be “probable AOM”. And if it is accompanied by a recent catarrh of the upper respiratory tracts, along with factors indicating a poor prognosis (AOM in a child younger than six months, relapsing or recurrent AOM, first-degree family history of middle ear sequelae due to AOM) probable AOM will be treated as a “confirmed AOM” (Fig. 1).
 TREATMENT OF ACUTE OTITIS MEDIA
TREATMENT OF ACUTE OTITIS MEDIA
Symptom management
The treatment of choice following diagnosis is pain relief (IA)25. Oral treatment with ibuprofen or paracetamol at the usual doses is usually enough, although ibuprofen has shown better results due to its double analgesic and anti-inflammatory activity1. If there is no response to treatment and the pain is intense, the practitioner must consider doing a tympanocentesis19.
Antibiotic treatment or the watchful waiting approach
In the past few years, it was been debated whether all AOM cases must be treated with antibiotics. These medications have been prescribed broadly with two purposes: to prevent complications and to improve symptoms. The most frequent severe complication is mastoiditis26, whose rates have dropped drastically with the use of antibiotic therapy27. A large-scale study on mastoiditis following AOM28 shows that AOM evolves into mastoiditis in 3.8 cases out of 10,000 episodes, versus 1.8 when the patient was treated with antibiotics28. 4,831 cases of AOM have to be treated to prevent a single case of mastoiditis. However, there has been a recent increase in the incidence of acute mastoiditis, probably related to the epidemiological changes that have occurred in pneumococcal strains in the past few years29,30, although not every author has observed this increase1. The effect of antimicrobial therapy on other rare complications, such as facial paralysis, labyrinthitis, and meningitis is not known. When it comes to the mildest complication, which is otitis media with effusion, treatment with antibiotics has not shown any long-term benefits32.
On the other hand, in 90% of the cases AOM can be considered a self-limiting disease. Numerous studies have demonstrated that most AOM cases treated solely for pain relief have good outcomes, although this depends to a great extent on the pathogenic agent. AOM caused by M. catarrhalis resolves spontaneously in more than 75% of the cases, as opposed to 50% of the cases caused by H. influenzae and 17% of the cases caused by pneumococcus13. Since only a small percentage of AOM cases result in complications, the goal should be the early treament of the small sub-group of children that tend to have poor outcomes or have risk factors.
As for the problem of the widespread use of antibiotics in children, it must be taken into account that AOM has been the most frequent cause of antibiotic prescription in children, significantly contributing to the increased resistance rates of respiratory tract pathogens33, so their use should be restricted as much as possible. The last reports on antimicrobial resistance in Spain, especially for respiratory tract pathogens, show decreased rates that are partly due to a more rational use of empirical antimicrobial therapy16.
All the reasons explained above, along with the side effects of antibiotics, support the current watchful waiting approach in response to a diagnosis of AOM, and deferring antibiotic treatment for cases with poor outcomes (those who have not improved in 48-72 hours)34-37 (IA). When taking this approach, the physician must be sure that he will be able to follow up with the patient1,19,25.
Groups at risk for a poor outcome: immediate antibiotic treatment
There is evidence (IA) that certain groups of children benefit from immediate antimicrobial treatment following diagnosis due to their higher risk for a poor outcome and the better response to antibiotic treatment shown in severe cases of AOM19,37-40:
- Children younger than two years, and especially those younger than six months, since they are at a higher risk to develop complications and have recurrent episodes. Furthermore, the rate of spontaneous recovery in this age group is low (AI)38.
- Children presenting with severe AOM (fever >39 ºC or acute pain), otorrhea or bilateral AOM41. It has been confirmed that these children benefit more from immediate antibiotic treatment (IA)37.
- Children with a history of recurrent or persistent AOM, or first-degree family members with ear sequelaes from inflammatory disease19.
Antibiotic selection
The practitioner must take into account which pathogen is most likely to be the cause of the disease and its level of antibiotic resistance. It is important that the antibiotic covers pneumococcus, since it is the microorganism that shows the lowest rate of spontaneous clearance and the highest rate of complications.
The first-line antibiotic is high-dosage amoxicillin (80-90 mg/kg a day divided in doses at 8 hour intervals) (IIB). At this dosage, this agent shows a strong bactericidal activity and reaches the middle ear in appropriate concentrations1,25,42.
However, in the past few years, following the introduction of conjugate pneumococcal vaccines, beta-lactamase producing non-typeable H. influenzae strains are becoming more important, especially in recurrent or persistent AOM43. Furthermore, it is estimated that one in every eight or nine otitis cases caused by this bacterium will not respond to treatment with amoxicillin44,45. Therefore, in children at risk for a poor outcome where the full spectrum of probable pathogens ought to be covered25, as well as in cases of amoxicillin treatment failure, the first line of treatment should be amoxicillin-clavulanic acid (8:1) in doses of 80-90 mg/kg/day of amoxicillin.
In short, amoxicillin-clavulanic acid (8:1) would be indicated in the following cases (IIB):
- Children younger than six months.
- Severe presentation in children younger than two years.
- Family history of sequelae due to frequent AOM.
- Amoxicillin treatment failure.
According to our current knowledge base, both amoxicillin and amoxicillin-clavulanic acid are preferably administered three times a day. However, in situations of poor therapeutic compliance, or when the circumstances of the patient so require, they can be administered every 12 hours, since the pharmacokynetics of amoxicillin allow the maintenance of high concentrations of the agent at the focus of infection when doses are given at 12-hour intervals46.
Other antibiotics
Cephalosporins, and especially cefuroxime axetyl, cover the entire spectrum except for penicillin-resistance pneumococcal strains, so they provide an alternative in cases of non-anaphylactic penicillin allergy40. If the patient cannot tolerate oral medications at the beginning of treatment, a dose of intramuscular ceftriaxone can be given at 50 mg/kg a day, continuing treatment with the oral preparation after 24 hours25. In case the gastric intolerance persists, the daily dosage of ceftriaxone can be maintained up to three days, which would complete the treatment.
The rates of macrolide-resistant pneumococci are increasingly high, up to 30-50% in Spain16, so these medications should not be used for treatment except in patients with a severe (Type I) penicillin allergy.
Duration of treatment
Traditionally, a long course of treatment has been recommended for AOM, lasting seven to ten days. However, some studies have proven that a short five-day course can be used in non-severe AOM cases in children older than two years with no risk factors47 (IA). The ten-day treatment course must be completed in children younger than six months, in severe cases of AOM, if there is a history of recurring AOM, or if there is an early recurrence of the symptoms (persistent AOM)19.
Acute Otitis Media Treatment Protocol
- Children younger than two months (IIIC): AOM is considered a severe disease in these children due to the high risk for complications, the relative immunosuppresion of the host, and the possibility of there being more than one pathogen causing the disease (gram-negative pathogen infection48). Admission to the hospital is recommended, and whenever possible, performance of a tympanocentesis to obtain a sample of otic exudate for culturing42.
- If the child presents with fever or a poor general health status, he will be treated intravenously with cefotaxime or amoxicillin-clavulanic acid at the standard dosage, and treatment will continue with oral preparations once his condition has improved.
- If the aforementioned symptoms are not present, treatment will be done with amoxicillin-clavulanic acid administered orally at high doses, and the patient will be kept under observation for two or three days, until discharged.
- Children aged two to six months (IA): this group has the highest risk for complications and recurrent AOM. Amoxicillin-clavulanic acid is indicated at 80-90 mg/kg a day, divided into 2-3 doses, for ten days.
- Children between six months and two years of age:
- A certain diagnosis of AOM indicates treatment with antibiotics from the beginning (IA). If the symptoms are mild to moderate, amoxicillin will be prescribed at 80-90 mg/kg per day for seven to ten days, divided in two or three doses. If the symptoms are severe, treatment will begin with high-dosage amoxicillin with clavulanic acid.
- If the diagnosis is unclear, antibiotic treatment will be started in patients with risk factors (recurring AOM, family history of AOM) or severe presentations. In the rest of cases, a follow-up evaluation will be performed in 24-48 hours.
- Children older than two years of age:
- If the presentation is severe or there are risk factors, the treatment will consist of amoxicillin at 80-90 mg/kg per day, divided in two or three doses, for 7-10 (IA)41.
- If the presentation is mild (fever <39 ºC, light pain) and there are no individual or family risk factors, it is recommended that the child is treated for pain relief and re-evaluated within 48 hours. If the symptoms persist or worsen, antibiotic therapy will be started with amoxicillin with a daily dosage of 80 mg/kg, and maintained for at least five days (IIIC).
- Treatment failure (IIIC): the treatment is considered to have failed when the clinical presentation has not improved 48-72 hours after starting treatment with antibiotics. The response will be to change the antibiotic agent3,25:
- If the original agent was amoxicillin, it will be replaced with amoxicillin-clavulanic acid (8:1) at 80-90 mg/kg a day divided in two or three doses.
- If it was amoxicillin-clavulanic acid (8:1), intramuscular ceftriaxone will be administered in single daily doses of 50 mg/kg for three days (AI). Ceftriaxone is a hospital-only drug, so the treatment must be done at the hospital.
- If treatment with ceftriaxone fails, the case must be overseen by the Otorhinolaryngology Department. A fluid sample will be taken by tympanocentesis for culture and antibiotic susceptibility testing, and the treatment will be determined according to the antibiogram (IIIC).
- Penicillin allergy:
- If there is a history of non-anaphylactic allergic reaction: cefuroxime axetyl at 30 mg/kg a day divided in two doses25,38 (IIIC).
- If there is a history of severe allergy with anaphylaxis: clarithromycin at 15 mg/kg a day in two doses for seven days, or azithromycin at 10 mg/kg in a single dose the first day, followed by a single daily dose of 5 mg/kg for four additional days, keeping the patient under strict observation since it is possible that the condition will evolve poorly. If the latter were the case, treatment of the patient will be transferred to the Otorhinolaryngology Department, a tympanocentesis will be done, and treatment will be determined by the antibiogram. If treatment with macrolides fails, one alternative is the oral administration of levofloxacin (IIIC) in 10 mg/kg doses given every twelve hours in chidren aged six months to five years, and in 10 mg/kg doses every 24 hours in children older than five years (the maximum dose is 500 mg)49,50. It must be taken into consideration that there is no oral syrup levofloxacin preparation (it is advised that the practitioner explains to the family the reasons this drug is indicated and appropriate). Ciprofloxacin is not indicated in AOM due to its weak activity against pneumococci.
CONFLICTS OF INTEREST
Fernando del Castillo, Fernando Baquero, and Cristina Calvo have participated in the Heracles Study, sponsored by Pfizer. Fernando Baquero has participated as a speaker in conferences sponsored by Pfizer and GSK. The rest of the authors report no conflicts of interest.
ACRONYMS: AEPAP: Spanish Association of Primary Care Pediatrics • AOM: acute otitis media • SAUCE: susceptibility to antimicrobial agents used in the Spanish community • SEIP: Spanish Society of Pediatric Infectology • SEPEAP: Spanish Society of Outpatient and Primary Care Pediatrics • SEUP: Spanish Society of Pediatrics Emergency Care.
BIBLIOGRAFHY
- Baquero-Artigao F, Del Castillo F. La otitis media aguda en la era de la vacunación antineumocócica. Enferm Infecc Microbiol Clin. 2008;26:505-9.
- Garbutt J, Jeffe DB, Shackelford P. Diagnosis and treatment of acute otitis media: an assessment. Pediatrics. 2003;112:143-9.
- del Castillo F, García-Perea A, Baquero-Artigao F. Bacteriology of acute otitis media in Spain: a prospective study based on tympanocentesis. Pediatr Infect Dis J. 1996;15:541-3.
- Dagan R, Givon-Lavi N, Zamir O, Sikuler-Cohen M, Guy L, Janco J, et al. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J Infect Dis. 2002;185:927-36.
- Bogaert D, Veenhoven RH, Sluijter M, Wannet WJW, Rijkers GT, Mitchell TJ, et al. Molecular epidemiology of pneumococcal colonization in response to pneumococcal conjugate vaccination in children with recurrent acute otitis media. J Clin Microbiol. 2005;43:74-83.
- Frazão N, Brito-Avô A, Simas C, Saldanha J, Mato R, Nunes S, et al. Effect of the seven-valent conjugate pneumococcal vaccine on carriage and drug resistance of Streptococcus pneumoniae in healthy children attending day-care centers in Lisbon. Pediatr Infect Dis J. 2005;24:243-52.
- Revai K, McCormick DP, Patel J, Grady JJ, Saeed K, Chonmaitree T. Effect of pneumococcal conjugate vaccine on nasopharyngeal bacterial colonization during acute otitis media. Pediatrics. 2006;117:1823-9.
- Fenoll A, Aguilar L, Vicioso MD, Giménez MJ, Robledo O, Granizo JJ. Increase in serotype 19A prevalence and amoxicillin non-susceptibility among paediatric Streptococcus pneumoniae isolates from middle ear fluid in a passive laboratory-based surveillance in Spain, 1997-2009. BMC Infect Dis. 2011;11:239.
- Block SL, Hedrick J, Harrison CJ, Tyler R, Smith A, Findlay R, et al. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J. 2004;23:829-33.
- Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr Infect Dis J. 2004;23:824-8.
- Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367:740-8.
- Cohen R, Levy C, Bingen E, Koskas M, Nave I, Varon E. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal nasopharyngeal carriage in children with acute otitis media. Pediatr Infect Dis J. 2012;31:297-301.
- Pichichero ME. Assesing the treatment alternatives for acute otitis media. Pediatr Infect Dis J. 1994;13:S27-S34.
- Klein JO, Teele DW. Isolation of viruses and mycoplasmas from middle ear effusions: a review. Ann Otol Rhinol Laryngol. 1976;85(Suppl 25):140-4.
- Ruuskanen O, Arola M, Heikkinen T, Ziegler T. Viruses in acute otitis media: increasing evidence for clinical significance. Pediatr Infect Dis J. 1991;10:425-7.
- Pérez-Trallero E, Martín-Herrero JE, Mazón A, García-Delafuente C, Robles P, Iriarte V, et al. Antimicrobial resistance among respiratory pathogens in Spain: latest data and changes over 11 years (1996-1997 to 2006-2007). Antimicrob Agents Chemother. 2010;54:2953-9.
- Picazo J, Ruiz-Contreras J, Casado-Flores J, Giangaspro E, del Castillo F, Hernández-Sampelayo T, et al.; Heracles Study Group. Relationship between serotypes, age, and clinical presentation of invasive pneumococcal disease in Madrid, Spain, after introduction of the 7-valent pneumococcal conjugate vaccine into the vaccination calendar. Clin Vaccine Immunol. 2011;18(1):89-94.
- García-Cobos S, Campos J, Cercenado E, Román F, Lázaro E, Pérez-Vázquez M, et al. Antibiotic resistance in Haemophilus influenzae decreased, except for beta-lactamase-negative amoxicillin-resistant isolates, in parallel with community antibiotic consumption in Spain from 1997 to 2007. Antimicrob Agents Chemother. 2008;52:2760-6.
- del Castillo F, Delgado A, Rodrigo C, Cervera J, Villafruela MA, Picazo JJ. Consenso nacional sobre otitis media aguda. An Pediatr (Barc). 2007;66:603-10.
- Castellarnau FE. Otitis. En: Benito FJ, Luances FC, Mintegui FS, Pou FJ. Tratado de urgencias en pediatría, 2.a ed. Madrid: Ergón; 2011. p. 309-33.
- del Castillo F, Baquero F, García MJ, Méndez A. Otitis media aguda. En: Protocolos diagnóstico-terapéuticos de la AEP: Infectología pediátrica. 2008. [en línea] [consultado el 23/04/2012]. Disponible en http://www.aeped.es/sites/default/files/documentos/oma.pdf
- Laine MK, Tähtinen PA, Ruuskanen O, Huovinen P, Ruohola A. Symptoms or symptom-based scores cannot predict acute otitis media at otitis-prone age. Pediatrics. 2010;125:e1154-61.
- Coker TR, Chan LS, Newberry SJ, Limbos MA, Suttorp MJ, Shekelle PG, et al. Diagnosis, microbial epidemiology, and antibiotic treatment of acute otitis media in children. A systematic review. JAMA. 2010;304:2161-9.
- Shaikh N, Hoberman A, Kaleida PH, Rockette HE, Kurs-Lasky M, Hoover H, et al. Otoscopic signs of otitis media. Pediatr Infect Dis J. 2011;30:822-6.
- American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451-65.
- Gower D, McGuirt WF. Intracranial complications of acute and chronic infectious ear disease: a problem still with us. Laryngoscope. 1983;93:1028-33.
- Migirov L, Duvdevani S, Kronenberg J. Otogenic intracranial complications. Acta Otolaryngol. 2005;125:819-22.
- Thompson PL, Gilbert RE, Long PF, Saxena S, Sharland M, Wong IC. Effect of antibiotics for otitis media on mastoiditis in children: a retrospective cohort study. Pediatrics. 2009;123:424-30.
- Dudkiewicz M, Livni G, Kornreich L, Nageris B, Ulanovski D, Raveh E. Acute mastoiditis and osteomyelitis of the temporal bone. Int J Pediatr Otorhinolaryngol. 2005;69:1399-405.
- Picazo J, Ruiz-Contreras J, Casado J, Giangaspro E, del Castillo F, Hernández-Sampelayo T, et al. Relationship between serotypes, age, and clinical presentation of invasive pneumococcal disease in Madrid, Spain, after introduction of the 7-valent pneumococcal conjugate vaccine into the vaccination calendar. Clin Vaccine Immunol. 2011;18:89-94.
- Groth A, Enoksson F, Hermansson A, Hultcrantz M, Stalfors J, Stenfeldt K. Acute mastoiditis in children in Sweden 1993-2007 - No increase after new guidelines. Int J Pediatr Otorhinolaryngol. 2011;75:1496-501.
- American Academy of Family Physicians; American Academy of Otolaryngology-Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media with Effusion. Otitis media with effusion. Pediatrics. 2004;113(5):1412-29.
- Picazo JJ, Betriu C, Rodríguez-Avial I, Azahares E, Ali Sánchez B. Vigilancia de resistencias a los antimicrobianos: estudio VIRA. Enferm Infecc Microbiol Clin. 2002;20:503-10.
- Stevanovic T, Komazec Z, Lemajic-Komazec S, Jovic R. Acute otitis media: to follow-up or treat? Int J Pediatr Otorhinolaryngol. 2010;74:930-3.
- Johnson NC, Holger JS. Pediatric acute otitis media: the case for delayed antibiotic treatment. J Emerg Med. 2007;32;279-84.
- McCormick DP, Chonmaitree T, Pittman C, Saeed K, Friedman NR, Uchida T, et al. Nonsevere acute otitis media: a clinical trial comparing outcomes of watchful waiting versus immediate antibiotic treatment. Pediatrics. 2005;115;1455-65.
- Rovers MM, Glasziou P, Appelman CL, Saeed K, Friedman NR, Uchida T, et al. Predictors of pain and/or fever at 3 to 7 days for children with acute otitis media non treated initially with antibiotics: a meta-analysis of individual patient data. Pediatrics. 2007;119:579-85.
- Hoberman A, Paradise JL, Rockette HE, Shaikh N, Wald ER, Kearney DH, et al. Treatment of acute otitis media in children under 2 years of age. N Engl J Med. 2011;364:105-15.
- Tahtinen PA, Laine MK, Huovinen P, Jalava J, Ruuskanen O, Ruohola A, et al. A placebo-controlled trial of antimicrobial treatment for acute otitis media. N Engl J Med. 2011;364:116-56.
- del Castillo F. Otitis media aguda: criterios diagnósticos y aproximación terapeútica. An Esp Pediatr. 2002;56 Suppl. 1:40-7.
- Marchissio P, Bellussi L, Di Mauro G, Doria M, Felisati G, Longhi R, et al. Acute otitis media: from diagosis to prevention. Summary of the Italian guideline. Int J Pediatr Otorhinolaryngol. 2010;74:1209-16.
- Gould JM, Matz PS. Otitis media. Pediatr Rev. 2010;31:102-16.
- Ito M, Hotomi M, Maruyama Y, Hatano M, Sugimoto H, Yoshizaki T, et al. Clonal spread of beta-lactamase-producing amoxicillin-clavulanate-resistant strains of non-typeable Haemophilus influenzae among young children attending a day care in Japan. Int J Pediatr Otorhinolaryngol. 2010;74:901-6.
- Klein JO. Antimicrobial therapy issues facing pediatricians. Pediatr Infect Dis J. 1995;14:415-19.
- Sox CM, Finkelstein JA, Yin R, Kleinman K, Lieu TA. Trends in otitis media treatment failure and relapse. Pediatrics. 2008;121:674-9.
- Isla A, Trocóniz IF, Canut A, Labora A, Martín-Herrero JE, Pedraz JL, et al. Evaluación farmacocinética/farmacodinámica de agentes antimicrobianos para el tratamiento de la otitis media aguda en España. Enferm Infecc Microbiol Clin. 2011;29:167-73.
- Kozyrskyj Al, Hildes-Ripstein GE, Longstaffe SE, Wincott JL, Sitar DS, Klassen TP, et al. Treatment of acute otitis media with a shortened course of antibiotics: a meta-analysis. JAMA.1998;279:1736-42.
- Berkun Y, Nir-Paz R, Ami AB, Klar A, Deutsch E, Hurvitz H. Acute otitis media in the first two months of life: characteristics and diagnostic difficulties. Arch Dis Child. 2008;93:690-4.
- Grimprel E, Cohen R. Levofloxacin in children. Arch Pediatr. 2010;17:S129-32.
- Moraga Llop FA, Cabañas Poy MJ. Guía de antiinfecciosos en pediatría. Madrid: Sanofi Pasteur MSD; 2010.





